Chinese Medical Sciences Journal ›› 2018, Vol. 33 ›› Issue (1): 29-37.doi: 10.24920/11802
黄芪甲苷拮抗Aβ1-42所致大鼠氧化应激,神经炎症和认知功能损伤
- 1陕西中医药大学病理教研室,陕西咸阳 712046
2西安交通大学附属西安市中心医院神经内科,西安 710003
3约克大学生物学系,加拿大 多伦多 M3J 1P3
4中国医学科学院 基础医学研究所 生物化学与分子生物学系 医学分子生物学国家重点实验室,北京 100005
-
收稿日期:2017-06-02出版日期:2018-02-13发布日期:2018-02-13 -
通讯作者:彭小忠 E-mail:pengxiaozhong@pumc.edu.cn
Astragaloside IV Protects Against Aβ1-42-induced Oxidative Stress, Neuroinflammation and Cognitive Impairment in Rats
Pan Yanfang1,Jia Xiaotao2,Song Erfei3,Peng Xiaozhong4,*( )
)
- 1Department of Pathology, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi 712046, China
2Department of Neurology, The Affiliated Xi'an Central Hospital of Xi'an Jiaotong University College of Medicine, Xi'an 710003, China;
3 Department of Biology, York University, Toronto, ON M3J 1P3, Canada
4State Key Laboratory of Medical Molecular Biology, Department of Molecular Biology and Biochemistry, Institute of Basic Medical Sciences Chinese Academy of Medical Sciences & School of Basic Medicine Peking Union Medical College, Beijing 100005, China;
-
Received:2017-06-02Published:2018-02-13Online:2018-02-13 -
Contact:Peng Xiaozhong E-mail:pengxiaozhong@pumc.edu.cn -
About author:This study investigated the neuroprotective action of astragaloside Ⅳon spatial learning and memory impairment induced by amyloid-beta 1-42 in rats and elucidated its underlying molecular mechanisms.
摘要: 目的 探讨黄芪甲苷拮抗淀粉样β蛋白(Aβ1-42)所致大鼠学习记忆损害的神经保护作用以及可能的分子机制。方法 成年Wistar雄性大鼠(体重230~250 g)被随机分为对照组、Aβ1-42、黄芪甲苷、(5,25和50 mg/kg·d)黄芪甲苷+Aβ1-42等6组。在脑立体定位仪引导下给大鼠侧脑室注射Aβ1-42。Aβ1-42 注射一周后进行Morris水迷宫实验(水下平台实验,空间探索实验,可见平台实验),以评估大鼠的空间学习记忆能力。Aβ1-42 注射后第8天开始腹腔注射黄芪甲苷(5,25和50 mg/kg·d), 连续注射5天。通过行为学软件记录大鼠寻找水下平台的平均逃避潜伏期、逃避距离、以及撤除平台后大鼠在目标象限内的游泳时间和距离百分比。同时测量大鼠的视力和游泳速度,以排除这些因素对记忆能力的影响。行为学实验后,大鼠被处死取出海马,然后测量不同处理组海马组织中的过氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GSH-px)和过氧化氢酶(CAT)活性。采用ELISA测量海马组织中白介素1β(IL-1β)及肿瘤坏死因子-α(TNF-α) 的水平。结果 水迷宫实验结果显示 :通过慢性给药,黄芪甲苷能够有效保护大鼠的学习记忆能力免受Aβ1-42损害。同时,黄芪甲苷能够有效拮抗Aβ1-42 所致大鼠海马组织SOD、GSH-px和CAT的活性下降。另外,黄芪甲苷能显著降低Aβ1-42激发的大鼠海马组织中IL-1β和TNF-α的水平。结论 黄芪甲苷能改善阿尔兹海默病模型大鼠的空间记忆能力、降低患者脑组织中的氧化应激和神经炎症反应水平。
引用本文
Pan Yanfang, Jia Xiaotao, Song Erfei, Peng Xiaozhong. Astragaloside IV Protects Against Aβ1-42-induced Oxidative Stress, Neuroinflammation and Cognitive Impairment in Rats[J].Chinese Medical Sciences Journal, 2018, 33(1): 29-37.

Figure 1.
Schematic diagram of drug treatment and behavioral tests.Aβ1-42 was injected into the intracerebroventricular of rats. After a recovery period for 7 days, AS-IV was intraperitoneally administrated at the doses of 5, 25 and 50 mg/kg·d respectively for 5 consecutive days. Aβ1-42: amyloid-beta 1-42; AS-IV: astragalosideIV."
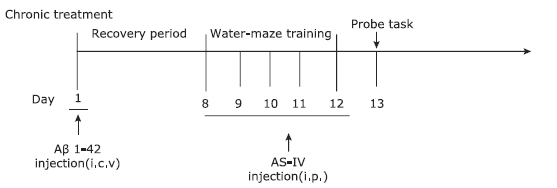

Figure 2.
AS-IV reatments attenuated Aβ1-42-induced spatial learning and memory impairment in rats. A. Rats with different treatments as labeled in the figure were trained for five consecutive days and the average escape latencies of rats were checked by the Morris water maze. B. The probe testing in various groups were performed four times per day and the percentages of total time in the target quadrant were calculated. C. Visible platform test was performed in rats with different treatments. D. The swimming speed (cm/s) in the various groups were evaluated. The data were represented as mean±SE. (n=10). *P<0.05 compared with the control group. #P<0.05, ##P<0.01 compared with the Aβ1-42 alone group."


Figure 3.
AS-IV attenuated the oxidative stress in the hippocampus of rats treated with Aβ1-42. Rats were received different treatments as labeled in the figure for five days and the activities of superoxide dismutase (SOD, A), glutathione peroxidase (GSH-px, B) and catalase (CAT, C) in hippocampus were checked. Values were expressed as mean ± SE (n=8). **P<0.01 compared with the control group; #P<0.05, ##P<0.01 compared with the Aβ1-42 group."


Figure 4.
AS-IV prevented the increase of IL-1β and TNF-α induced by Aβ1-42. ELISA for interleukin-1 beta (IL-1β, A) and tumor necrosis factor-alpha (TNF-α, B) were performed in the hippocampus tissue of rats with various treatments. Values are expressed as mean ± SE (n=8). **P<0.01 compared with the control group; #P<0.05, ##P<0.01 compared with the Aβ1-42 group."

| 1. |
Goedert M, Spillantini MG.A century of Alzheimer’s disease. Science 2006; 314(5800):777-81. doi: 10.1126/science.1132814.
doi: 10.1126/science.1132814 pmid: 17082447 |
| 2. |
Anand R, Gill KD, Mahdi AA. Therapeutics of Alzheimer’s disease: past, present and future Neuropharmacology 2014; 76 PtA:27-50. doi: 10.1016/j.neuropharm.2013.07.004.
doi: 10.1016/j.neuropharm.2013.07.004 pmid: 23891641 |
| 3. |
Esparza TJ, Zhao H, Cirrito JR, et al.Amyloid-beta oligomerization in Alzheimer dementia versus high-pathology controls. Ann Neurol 2013;73(1):104-19. doi: 10.1002/ana.23748.
doi: 10.1002/ana.23748 pmid: 3563737 |
| 4. |
Zetterberg H, Blennow K, Hanse E.Amyloid beta and APP as biomarkers for Alzheimer’s disease. Exp Gerontol 2010; 45(1):23-9. doi: 10.1016/j.exger.2009.08.002.
doi: 10.1016/j.exger.2009.08.002 |
| 5. |
Yatin SM, Yatin M, Aulick T, et al.Alzheimer’s amyloid beta-peptide associated free radicals increase rat embryonic neuronal polyamine uptake and ornithine decarboxylase activity: protective effect of vitamin E. NeurosciLett 1999; 263(1):17-20. doi: 10.1016/S0304-3940(99)00101-96.
doi: 10.1016/S0304-3940(99)00101-96 pmid: 10218900 |
| 6. |
Zotova E, Nicoll JA, Kalaria R, et al.Inflammation in Alzheimer’s disease: relevance to pathogenesis and therapy. Alzheimers Res Ther 2010; 2(1):1. doi: 10.1186/alzrt24.
doi: 10.1186/alzrt24 pmid: 2874260 |
| 7. |
Hickman SE, Allison EK, El KJ.Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci 2008; 28(33):8354-60. doi: 10.1523/JNEUROSCI.0616-08.2008.
doi: 10.1523/JNEUROSCI.0616-08.2008 pmid: 18701698 |
| 8. |
Johnston H, Boutin H, Allan SM.Assessing the contribution of inflammation in models of Alzheimer’s disease. Biochem Soc Trans 2011; 39(4):886-90. doi: 10.1042/BST0390886.
doi: 10.1042/BST0390886 pmid: 21787318 |
| 9. |
Godbout JP, Chen J, Abraham J, et al.Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J 2005; 19(10):1329-31. doi: 10.1096/fj.05-3776fj.
doi: 10.1096/fj.05-3776fj pmid: 15919760 |
| 10. |
Ullah F, Ali T, Ullah N, et al.Caffeine prevents d-galactose-induced cognitive deficits, oxidative stress, neuroinflammation and neurodegeneration in the adult rat brain. Neurochem Int 2015; 90:114-24. doi: 10.1016/j.neuint.2015.07.001.
doi: 10.1016/j.neuint.2015.07.001 pmid: 26209154 |
| 11. |
Tan CC, Yu JT, Wang HF, et al.Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 2014; 41(2):615-31. doi: 10.3233/JAD-132690.
doi: 10.3233/JAD-132690 pmid: 24662102 |
| 12. |
Saini SS, Gesselllee DL, Peterson JW.The cox-2-specific inhibitor celecoxib inhibits adenylyl cyclase.Inflammation 2003; 27(2):79-88. doi: 10.1023/A:1023226616526.
doi: 10.1023/A:1023226616526 pmid: 12797547 |
| 13. |
Fu J, Wang Z, Huang L, et al.Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother Res 2014; 28(9):1275-83. doi: 10.1002/ptr.5188.
doi: 10.1002/ptr.5188 pmid: 25087616 |
| 14. |
Zhang ZG, Wu L, Wang JL, et al.AstragalosideIV prevents MPP(+)-induced SH-SY5Y cell death via the inhibition of Bax-mediated pathways and ROS production. Mol Cell Biochem 2012; 364(1-2):209-16. doi: 10.1007/s11010-011-1219-1.
doi: 10.1007/s11010-011-1219-1 |
| 15. |
Li X, Wang X, Han C, et al.Astragaloside IV suppresses collagen production of activated hepatic stellate cells via oxidative stress-mediated p38 MAPK pathway. Free Radic Biol Med 2013; 60:168-76. doi: 10.1016/j.freeradbiomed.
doi: 10.1016/j.freeradbiomed pmid: 23459070 |
| 16. |
Hu JY, Han J, Chu ZG, et al.AstragalosideIVattenuates hypoxia-induced cardiomyocyte damage in rats by upregulating superoxide dismutase-1 levels. Clin Exp Pharmacol Physiol 2009; 36(4):351-7. doi: 10.1111/j.1440-1681.2008.05059.x.
doi: 10.1111/j.1440-1681.2008.05059.x pmid: 18986331 |
| 17. |
Li M, Qu YZ, Zhao ZW, et al.AstragalosideIVprotects against focal cerebral ischemia/reperfusion injury correlating to suppression of neutrophils adhesion-related molecules. Neurochem Int 2012; 60(5):458-65. doi: 10.1016/j.neuint.2012.01.026.
doi: 10.1016/j.neuint.2012.01.026 pmid: 22342823 |
| 18. |
Liu G, Song J, Guo Y, et al.Astragalus injection protects cerebral ischemic injury by inhibiting neuronal apoptosis and the expression of JNK3 after cerebral ischemia reperfusion in rats. Behav Brain Funct 2013; 9:36. doi: 10.1186/1744-9081-9-36.
doi: 10.1186/1744-9081-9-36 pmid: 24083559 |
| 19. |
Kim S, Kang IH, Nam JB, et al.Ameliorating the effect of astragalosideIVon learning and memory deficit after chronic cerebral hypoperfusion in rats. Molecules 2015; 20(2):1904-21. doi: 10.3390/molecules20021904.
doi: 10.3390/molecules20021904 pmid: 25625683 |
| 20. |
Paranjape GS, Terrill SE, Gouwens LK, et al.Amyloid-β(1-42) protofibrils formed in modified artificial cerebrospinal fluid bind and activate microglia. J Neuroimmune Pharmacol 2013; 8(1):312-22. doi: 10.1007/s11481-012-9424-6.
doi: 10.1007/s11481-012-9424-6 pmid: 23242692 |
| 21. |
Nakamura S, Murayama N, Noshita T, et al.Progressive brain dysfunction following intracerebroventricular infusion of beta (1-42)-amyloid peptide. Brain Res 2001; 912(2):128-36. doi: 10.1016/S0006-8993(01)02704-4.
doi: 10.1016/S0006-8993(01)02704-4 pmid: 11532428 |
| 22. |
Pan YF, Chen XR, Wu MN, et al.Arginine vasopressin prevents against Aβ25-35-induced impairment of spatial learning and memory in rats. Horm Behav 2010; 57(4-5):448-54. doi: 10.1016/j.yhbeh.2010.01.015.
doi: 10.1016/j.yhbeh.2010.01.015 pmid: 20138885 |
| 23. |
Butterfield DA, Boyd-Kimball D. Amyloid β-peptide(1-42) contributes to the oxidative stress and neurodegeneration found in Alzheimer disease brain. Brain Pathol 2004; 14(4):426-32. doi: 10.1111/j.1750-3639.2004.tb00087.x.
doi: 10.1111/j.1750-3639.2004.tb00087.x pmid: 15605990 |
| 24. |
Praticò D.Oxidative stress hypothesis in Alzheimer’s disease: a reappraisal. Trends Pharmacol Sci 2008; 29(12):609-15. doi: 10.1016/j.tips.2008.09.001.
doi: 10.1016/j.tips.2008.09.001 pmid: 18838179 |
| 25. |
Kamer A, Craig RG, Dasanayake AP, et al.Inflammation and Alzheimer’s disease: possible role of periodontal diseases. Alzheimers Dement 2008; 4(4):242-50. doi: 10.1016/j.jalz.2007.08.004.
doi: 10.1016/j.jalz.2007.08.004 pmid: 18631974 |
| 26. |
Cai HY, Holscher C, Yue XH, et al.Lixisenatide rescues spatial memory and synaptic plasticity from amyloid β protein-induced impairments in rats. Neuroscience 2014; 277:6-13. doi: 10.1016/j.neuroscience.2014.02.022.
doi: 10.1016/j.neuroscience.2014.02.022 pmid: 24583037 |
| 27. |
Klein WL, Jr SW, Teplow DB.Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer’s disease. Neurobiol Aging 2004; 25(5):569-80. doi: 10.1016/j.neurobiolaging.2004.02.010.
doi: 10.1016/j.neurobiolaging.2004.02.010 pmid: 15172732 |
| 28. |
Nillert N, Pannangrong W, Welbat JU, et al. Neuroprotective effects of aged garlic extract on cognitive dysfunction and neuroinflammation induced by β-amyloid in rats. Nutrients2017; 9(1). pii: E24. doi: 10.3390/nu9010024.
doi: 10.3390/nu9010024 |
| 29. |
Wang J, Ho L, Zhao W, et al.Grape-derived polyphenolics prevent Aβ oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J Neurosci 2008; 28(25):6388-92. doi: 10.1523/JNEUROSCI.0364-08.2008.
doi: 10.1523/JNEUROSCI.0364-08.2008 pmid: 18562609 |
| 30. |
Dong Z, Bai Y, Wu X, et al.Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology 2013; 64:65-73. doi: 10.1016/j.neuropharm.2012.06.027.
doi: 10.1016/j.neuropharm.2012.06.027 |
| 31. |
Jia XT, Ye-Tian, Yuan-Li, et al.Exendin-4, a glucagon-like peptide 1 receptor agonist, protects against amyloid-β peptide-induced impairment of spatial learning and memory in rats. Physiol Behav 2016; 159:72-9.doi: 10.1016/j.physbeh. 2016.03.016.
doi: 10.1016/j.physbeh. 2016.03.016 pmid: 26992957 |
| 32. |
Wan L, Nie G, Zhang J, et al.β-Amyloid peptide increases levels of iron content and oxidative stress in human cell and Caenorhabditis elegans models of Alzheimer disease. Free Radic Biol Med 2011; 50(1):122-9. doi: 10.1016/j.freeradbiomed.2010.10.707.
doi: 10.1016/j.freeradbiomed.2010.10.707 pmid: 21034809 |
| 33. |
Abdul HM, Sultana R, St Clair DK, et al.Oxidative damage in brain from human mutant APP/PS-1 double knock-in mice as a function of age. Free Radic Biol Med 2008; 45(10):1420-5. doi: 10.1016/j.freerad biomed.2008.08.012.
doi: 10.1016/j.freerad biomed.2008.08.012 pmid: 2597707 |
| 34. |
Chen H, Yoshioka H, Kim GS, et al.Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal 2011; 14(8):1505-17. doi: 10.1089/ars.2010.3576.
doi: 10.1089/ars.2010.3576 pmid: 20812869 |
| 35. |
Pong K, Rong Y, Doctrow SR, et al.Attenuation of zinc-induced intracellular dysfunction and neurotoxicity by a synthetic superoxide dismutase/catalase mimetic, in cultured cortical neurons. Brain Res 2002; 950(1-2):218-30. doi:10.1016/S0006-8993(02)03040-8.
doi: 10.1016/S0006-8993(02)03040-8 pmid: 12231247 |
| 36. |
Hofmann U, Heuer S, Meder K, et al.The proinflammatory cytokines TNF-alpha and IL-1 beta impair economy of contraction in human myocardium. Cytokine 2007; 39(3):157-62. doi: 10.1016/j.cyto.2007.07.185.
doi: 10.1016/j.cyto.2007.07.185 pmid: 17825578 |
| 37. |
Detloff MR, Fisher LC, Mcgaughy V, et al.Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol 2008; 212(2):337-47. doi: 10.1016/j.expneurol.2008.04.009.
doi: 10.1016/j.expneurol.2008.04.009 pmid: 18511041 |
| 38. |
Rogers JT, Leiter LM, Mcphee J, et al.Translation of the alzheimer amyloid precursor protein mRNA is up-regulated by interleukin-1 through 5’-untranslated region sequences. J Biol Chem 1999; 274(10):6421-31. doi: 10.1074/jbc.274.10.6421.
doi: 10.1074/jbc.274.10.6421 |
| [1] | 修建波, 李岚岚, 许琪. 米诺环素活化孤束核相关网络减轻脂多糖诱发的神经炎症[J]. Chinese Medical Sciences Journal, 2022, 37(1): 1-14. |
| [2] | 平芬, 曹芹, 林桦, 韩书芝. N-乙酰半胱氨酸对PM2.5致大鼠肺损伤时MAPK主要通路蛋白活化及氧化炎症反应的影响[J]. Chinese Medical Sciences Journal, 2019, 34(4): 270-276. |
| [3] | 蔡萃,徐长青,金华良,厉蓓. 慢性阻塞性肺疾病伴抑郁对老年大鼠空间学习记忆能力的影响[J]. Chinese Medical Sciences Journal, 2018, 33(4): 260-266. |
| [4] | 何颖,张迎,王梦莹,张萌,张丹,张莹,蒋卓澄,吴锋,陈静. 筛选双氧水引起肥大软骨细胞中发生改变的基因[J]. Chinese Medical Sciences Journal, 2018, 33(1): 45-52. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
|

