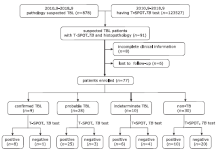
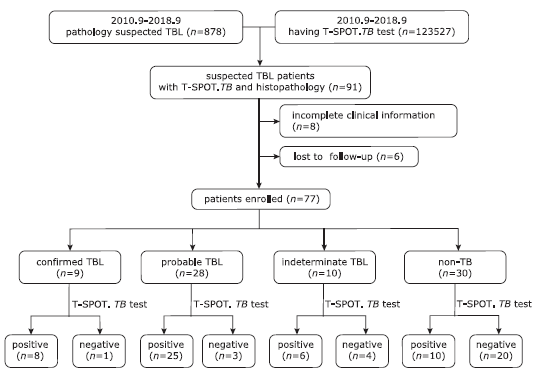
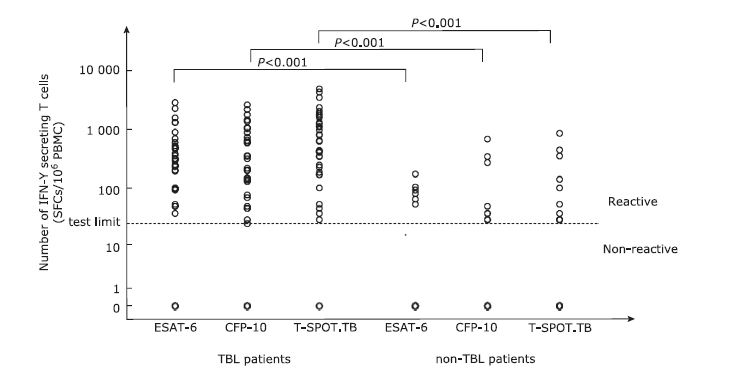
| 1. |
World Health Organization. Global Tuberculosis Report 2019. Geneva: World Health Orgernization; 2019. P27. Licence: CC BY-NC-SA 3.0 IGO.
|
| 2. |
Pehme L, Hollo V, Rahu M , et al. Tuberculosis during fundamental societal changes in Estonia with special reference to extrapulmonary manifestations. Chest 2005; 127(4):1289-95. doi: 10.1016/S0012-3692(15)34479-2.
|
| 3. |
Liao CH, Chou CH, Lai CC , et al. Diagnostic performance of an enzyme-linked immunospot assay for interferon-gamma in extrapulmonary tuberculosis varies between different sites of disease. J Infect 2009; 59(6):402-8. doi: 10.1016/j.jinf.2009.10.001.
|
| 4. |
Song KH, Jeon JH, Park WB , et al. Usefulness of the whole-blood interferon-gamma release assay for diagnosis of extrapulmonary tuberculosis. Diagn Microbiol Infect Dis 2009; 63(2):182-7. doi: 10.1016/j.diagmicrobio.2008.10.013.
|
| 5. |
Benjelloun A, Darouassi Y, Zakaria Y , et al. Lymph nodes tuberculosis: a retrospective study on clinical and therapeutic feature. Pan African Med J 2015; 20:65. doi: 10.11604/pamj.2015.20.65.5782.
|
| 6. |
Shin JA, Chang YS, Kim HJ , et al. Diagnostic utility of interferon-gamma release assay in extrapulmonary tuberculosis. Diagn Microbiol Infect Dis 2015; 82(1):44-8. doi: 10.1016/j.diagmicrobio.2015.02.002.
|
| 7. |
Kim KH, Kim RB, Woo SH , et al. The efficacy of the interferon-γ release assay for diagnosing cervical tuberculous lymphadenitis: a prospective controlled study. Laryngoscope 2016; 126:378-84. doi: 10.1002/lary.25540.
|
| 8. |
Kim YK, Uh Y, Lee NS , et al. Whole-blood interferon-gamma release assay for diagnosis of tuberculous lymphadenitis. Tohoku J Exp Med 2011; 224(3):189-93. doi: 10.1620/tjem.224.189.
|
| 9. |
Kim JK, Ko JJ, Kim KC , et al. Comparison of the tuberculin skin test and the interferon-γ release assay for the diagnosis of cervical tuberculous lymphadenitis. Korean J Urol 2013; 56(6):354-8. doi: 10.3342/kjorl-hns.2013.56.6.354.
|
| 10. |
Jia H, Pan L, Du B , et al. Diagnostic performance of interferon-γ release assay for lymph node tuberculosis. Diagn Microbiol Infect Dis 2016; 85(1):56-60. doi: 10.1016/j.diagmicrobio.2016.02.001.
|
| 11. |
Lai CC, Liao CH, Tan CK , et al. Diagnosis of peripheral tuberculous lymphadenitis by enzyme-linked immunospot assay for interferon-gamma. Am J Med 2009; 122(8):e3. doi: 10.1016/j.amjmed.2009.02.011.
|
| 12. |
Cho OH, Park KH, Kim SM , et al. Diagnostic performance of T-SPOT.TB for extrapulmonary tuberculosis according to the site of infection. J Infect 2011; 6(3):362-9. doi: 10.1016/j.jinf.2011.06.010.
|
| 13. |
Lu X, Li WP, Xie YH , et al. Evaluation of interferon-gamma release technique in extra-pulmonary tuberculosis detection. Chin J Lung Dis 2016; 1(9):20-5. Chinese. doi: 10.3877/cma.j.issn.1674-6902.2016.01.006.
|
| 14. |
Diel R, Loddenkemper R, Nienhaus A . Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a meta analysis. Chest 2010; 137(4):952-68. doi: 10.1378/chest.09-2350.
|
| 15. |
Sadatsafavi M, Shahidi N, Marra F , et al. A statistical method was used for the meta-analysis of tests for latent TB in the absence of a gold standard, combining random-effect and latent-class methods to estimate test accuracy. J Clin Epidemiol 2010; 63(3):257-69. doi: 10.1016/j.jclinepi.2009.04.008.
|
| 16. |
Cambau E, Drancourt M . Steps towards the discovery of Mycobacterium tuberculosis by Robert Koch, 1882. Clin Microbiol Infect 2014; 20(3):196-201. doi: 10.1111/1469-0691.12555.
|
| 17. |
Haldar S, Bose M, Chakrabarti P , et al. Improved laboratory diagnosis of tuberculosis-the Indian experience. Tuberculosis 2011; 91(5):414-26. doi: 10.1016/j.tube.2011.06.003.
|
| 18. |
Verma JS, Dhavan I, Nair D , et al. Rapid culture diagnosis of tuberculous lymphadenitis from a tertiary care centre in an endemic nation: potential and pitfalls. Indian J Med Microbiol 2012; 30:342-5. doi: 10.4103/0255-0857.99498.
|
| 19. |
Agarwal AK, Sethi A, Sethi D , et al. Tubercular cervical adenitis: clinicopathologic analysis of 180 cases. J Otolaryngol Head Neck Surg 2009; 38(5):521-5. doi: 10.2310/7070.2009.080212.
|
| 20. |
Sinha P, Prakash P, Patne SC , et al. Performance of nested multiplex PCR assay targeting MTP40 and IS6110 gene sequences for the diagnosis of tubercular lymphadenitis. J Microbiol 2017; 55(1):63-7. doi: 10.1007/s12275-017-6127-y.
|
| 21. |
Ikram A, Ahmed SA, Khan FA , et al. Rapid Mycobacterium tuberculosis DNA detection on fine needle aspirates from extra pulmonary lymph nodes. J Coll Physicians Surg Pak 2015; 25(6):417-21. doi: 06.2015/JCPSP.417421.
|
| 22. |
Mittal P, Handa U, Mohan H , et al. Comparative evaluation of fine needle aspiration cytology, culture, and PCR in diagnosis of tuberculous lymphadenitis. Diagn Cytopathol 2011; 39(11):822-6. doi: 10.1002/dc.21472.
|
| 23. |
Gillissen A . Tuberculosis-epidemiology, diagnostics and therapy. MMW Forts chr Med 2016; 158(6):50-5. doi: 10.1007/s15006-016-7650-1.
|
| 24. |
Neonakis IK, Spandidos DA, Petinaki E . Female genital tuberculosis: a review. Scand J Infect Dis 2011; 43:564-72. doi: 10.3109/00365548.2011.568523.
|
| 25. |
Jia HY, Pan LP, Liu F. Adjunctive diagnostic value of interferon-gamma release assay in tuberculosis lymphadenitis. Chin J Antituberculosis 2014; 36(6):467-71. Chinese. doi: 10.3969/j.issn.1000-6621.2014.06.012.
|
| 26. |
Pan L, Jia H, Liu F , et al. Risk factors for false-negative T-SPOT.TB assay results in patients with pulmonary and extra-pulmonary TB. J Infect 2015; 70(4):367-80. doi: 10.1016/j.jinf.2014.12.018.
|
| 27. |
Hang NT, Lien LT, Kobayashi N , et al. Analysis of factors lowering sensitivity of interferon-gamma release assay for tuberculosis. PLoS One 2011; 6:e23806. doi: 10.1371/journal.pone.0023806.
|
| 28. |
Gao L, Lu W, Bai L , et al. Latent tuberculosis infection in rural China: baseline results of a population-based, multicentre, prospective cohort study. Lancet Infect Dis 2015; 15(3):310-19. doi: 10.1016/S1473-3099(14)71085-0.
|
| 29. |
Liu XQ, Bian SN, Cheng XH , et al. Utility of T-cell interferon-γ release assays for the diagnosis of female genital tuberculosis in a tertiary referral hospital in Beijing, China. Medicine 2016; 95(44):e5200. doi: 10.1097/md.0000000000005200.
|
| 30. |
Wang LC, Yu Y, Chen W , et al. Evaluation of the characteristics of the enzyme-linked immunospot assay for diagnosis of active tuberculosis in China. Clin Vaccine Immunol 2015; 22:510-5. doi: 10.1128/CVI.00023-15.
|
| 31. |
Temple SE, Pham K, Glendenning P , et al. Endotoxin induced TNF and IL-10 mRNA production is higher in male than female donors: correlation with elevated expression of TLR4. Cell Immunol 2008; 251(2):69-71. doi: 10.1016/j.cellimm.2008.04.013.
|
| 32. |
Chan YM, Noertjojo K, Chan SL , et al. Sex differences in tuberculosis in Hong Kong. Int J Tuberc Lung Dis 2002; 6(1):11-8. doi: 10.1067/mhl.2002.120259.
|
 )
)