Chinese Medical Sciences Journal ›› 2018, Vol. 33 ›› Issue (1): 1-8.doi: 10.24920/11805
• Original Articles • Next Articles
Recombinant Expression and Purification of Mouse Nectin-like 4 Glycoprotein in 293ET Cell Line
Li Dongdong, An Tai, Liu Xiao, Yin Bin, Peng Xiaozhong( ), Shu Pengcheng(
), Shu Pengcheng( )
)
- State Key Laboratory of Medical Molecular Biology, Institute of Basic Medical Sciences Chinese Academy of Medical Sciences & School of Basic Medicine Peking Union Medical College, Beijing 100005, China
-
Received:2017-10-23Published:2017-03-17Online:2018-03-07 -
About author:Corresponding author Tel: 86-10-69156434, Fax: 86-10-65240529; E-mail: Xiaozhong Pengpengxiaozhong@pumc.edu.cn |Corresponding author Tel: 86-10-69156434, Fax: 86-10-65240529; E-mail: Pengcheng Shupengcheng_shu@ibms.pumc.edu.cn
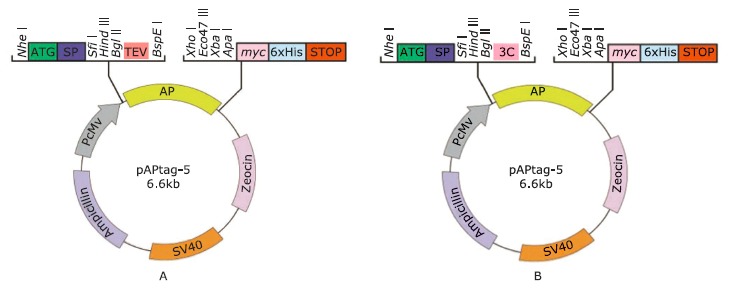
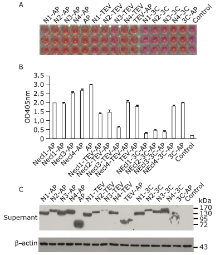
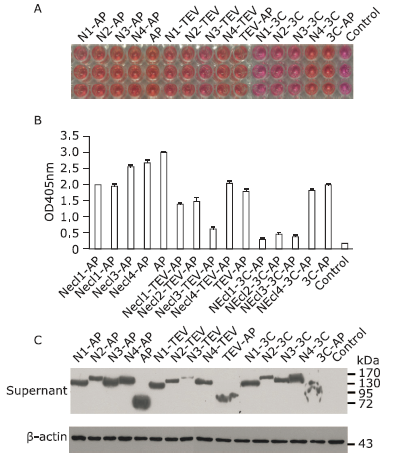
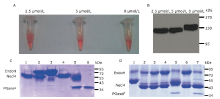
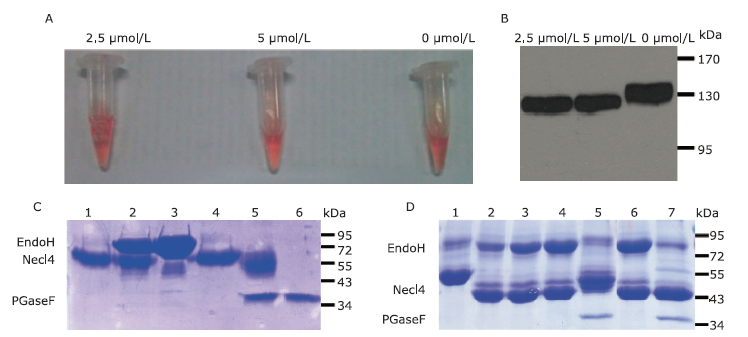
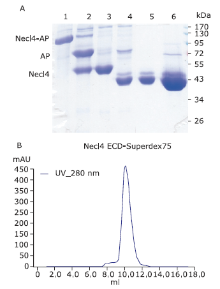
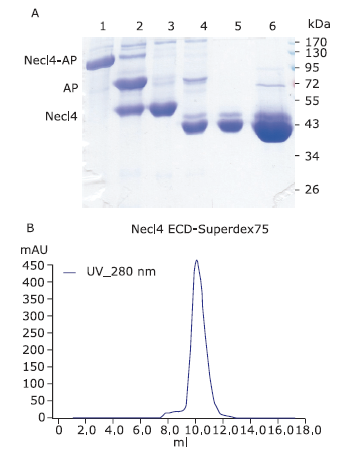
| The authors constructed a modified AP mammalian protein expression system to screen the high productive stable cell lines expressing Nectin-like 4 protein. By adding mannosidase inhibitor kifunensine into the medium and cutting purified protein by using endoglycosidase H, they obtained deglycosylated Necl-4 protein in milligram quantities. |
Cite this article
Li Dongdong, An Tai, Liu Xiao, Yin Bin, Peng Xiaozhong, Shu Pengcheng. Recombinant Expression and Purification of Mouse Nectin-like 4 Glycoprotein in 293ET Cell Line[J].Chinese Medical Sciences Journal, 2018, 33(1): 1-8.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
"
| Genes | 5’ primer | 3’ primer |
|---|---|---|
| mNecl-1 | 5’-CCCAAGCTTACATCTTTCCCAGGACGATAG-3’ | 5’-GAAGATCTGTGGTAGGTACTGGAGGAC-3’ |
| mNecl-2 | 5’-CCCAAGCTTACCAGAATCTGTTTACTAAAGACGTG-3’ | 5’-GAAGATCTGTGGTCCACTGCCCC-3’ |
| mNecl-3 | 5’-GAAGATCTCAGTTTCCACTAACTCAGAATG-3’ | 5’-GAAGATCTATGGTCAGGGCCATTCTG-3’ |
| mNecl-4 | 5’-CCCAAGCTTACCAGGAAGTACAGACCGAG-3’ | 5’-GAAGATCTGGCGTAAGGAACCGATG-3’ |
| 1. |
Samanta D, Almo SC . Nectin family of cell-adhesion molecules: structural and molecular aspects of function and specificity. Cell Mol Life Sci,2015; 72(4):645-58. doi: 10.1007/s00018-014-1763-4.
doi: 10.1007/s00018-014-1763-4 pmid: 25326769 |
| 2. |
Mandai K, Rikitake Y, Mori M, et al. Nectins and nectin-like molecules in development and disease. Curr Top Dev Biol,2015; 112:197-231. doi: 10.1016/bs.ctdb.2014.11.019.
doi: 10.1016/bs.ctdb.2014.11.019 |
| 3. |
Galibert L, Diemer GS, Liu Z, et al. Nectin-like protein 2 defines a subset of T-cell zone dendritic cells and is a ligand for class-I-restricted T-cell-associated molecule. J Biol Chem,2005; 280(23):21955-64. doi: 10.1074/jbc.M502095200.
doi: 10.1074/jbc.M502095200 |
| 4. |
Kakunaga S, Ikeda W, Itoh S, et al. Nectin-like molecule-1/TSLL1/SynCAM3: a neural tissue-specific immunoglobulin-like cell-cell adhesion molecule localizing at non-junctional contact sites of presynaptic nerve terminals, axons and glia cell processes. J Cell Sci,2005; 118(6):1267-77. doi: 10.1242/jcs.01656.
doi: 10.1242/jcs.01656 |
| 5. |
Mizutani K, Takai Y . Nectin spot: a novel type of nectin-mediated cell adhesion apparatus. Biochem J,2016; 473(18):2691-715. doi: 10.1042/BCJ20160235.
doi: 10.1042/BCJ20160235 pmid: 27621480 |
| 6. |
Catros V, Dessarthe B . Nectins and nectin-like recaeptors DNAM-1 and CRTAM: new ways for tumor escape. Med Sci,2014; 30(5):537-43. doi: 10.1051/medsci/20143005017
doi: 10.1051/medsci/20143005017 pmid: 24939541 |
| 7. |
Zhou Y, Du G, Hu X, et al. Nectin-like molecule 1 is a protein 4.1N associated protein and recruits protein 4.1N from cytoplasm to the plasma membrane. Biochim Biophys Acta,2005; 1669(2):142-54. doi: 10.1016/j.bbamem.2005.01.013.
doi: 10.1016/j.bbamem.2005.01.013 pmid: 15893517 |
| 8. |
Maurel P, Einheber S, Galinska J, et al. Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination. J Cell Biol,2007; 178(5):861-74. doi: 10.1083/jcb.200705132.
doi: 10.1083/jcb.200705132 pmid: 17724124 |
| 9. |
Spiegel I, Adamsky K, Eshed Y, et al. A central role for Necl4 (SynCAM4) in Schwann cell-axon interaction and myelination. Nat Neurosci,2007; 10(7):861-9. doi: 10.1038/nn1915.
doi: 10.1038/nn1915 pmid: 17558405 |
| 10. |
Park J, Liu B, Chen T, et al. Disruption of Nectin-like 1 cell adhesion molecule leads to delayed axonal myelination in the CNS. J Neurosci,2008; 28(47):12815-9. doi: 10.1523/JNEUROSCI.2665-08.2008.
doi: 10.1523/JNEUROSCI.2665-08.2008 pmid: 19036974 |
| 11. |
Heffernan C, Jain MR, Liu T, et al. Nectin-like 4 complexes with choline transporter-like protein-1 and regulates Schwann cell choline homeostasis and lipid biogenesis in vitro. J Biol Chem,2017; 292(11):4484-98. doi: 10.1074/jbc.M116.747816.
doi: 10.1074/jbc.M116.747816 pmid: 28119456 |
| 12. |
Zhu Y, Li H, Li K, et al. Necl-4/SynCAM-4 is expressed in myelinating oligodendrocytes but not required for axonal myelination. PLoS One,2013; 8(5):e64264. doi: 10.1371/journal.pone.0064264.
doi: 10.1371/journal.pone.0064264 |
| 13. |
Golan N, Kartvelishvily E, Spiegel I, et al. Genetic deletion of Cadm4 results in myelin abnormalities resembling Charcot-Marie-Tooth neuropathy. J Neurosci,2013; 33(27):10950-61. doi: 10.1523/JNEUROSCI.0571-13.2013.
doi: 10.1523/JNEUROSCI.0571-13.2013 |
| 14. |
Yang S, Weng H, Chen L, et al. Lack of protein 4.1G causes altered expression and localization of the cell adhesion molecule nectin-like 4 in testis and can cause male infertility. Mol Cell Biol,2011; 31(11): 2276-86. doi: 10.1128/MCB.01105-10.
doi: 10.1128/MCB.01105-10 |
| 15. |
Raveh S, Gavert N, Spiegel I, et al. The cell adhesion nectin-like molecules (Necl) 1 and 4 suppress the growth and tumorigenic ability of colon cancer cells. J Cell Biochem,2009; 108(1):326-36. doi: 10.1002/jcb.22258.
doi: 10.1002/jcb.22258 |
| 16. |
Dong X, Xu F, Gong Y, et al. Crystal structure of the V domain of human Nectin-like molecule-1/Syncam3/Tsll1/Igsf4b, a neural tissue-specific immunoglobulin-like cell-cell adhesion molecule. J Biol Chem,2006; 281(15):10610-7. doi: 10.1074/jbc.M513459200.
doi: 10.1074/jbc.M513459200 |
| 17. |
Fogel AI, Li Y, Giza J, et al. N-glycosylation at the SynCAM (synaptic cell adhesion molecule) immunoglobulin interface modulates synaptic adhesion. J Biol Chem,2010; 285(45):34864-74. doi: 10.1074/jbc.M110.120865.
doi: 10.1074/jbc.M110.120865 |
| 18. |
Liu J, Qian X, Chen Z, et al. Crystal structure of cell adhesion molecule nectin-2/CD112 and its binding to immune receptor DNAM-1/CD226. J Immunol,2012; 188(11):5511-20. doi: 10.4049/jimmunol.1200324.
doi: 10.4049/jimmunol.1200324 pmid: 22547693 |
| 19. |
Gao J, Chen T, Hu G, et al. Nectin-like molecule 1 is a glycoprotein with a single N-glycosylation site at N290KS which influences its adhesion activity. Biochim Biophys Acta,2008; 1778(6):1429-35. doi: 10.1016/j.bbamem.2008.03.013.
doi: 10.1016/j.bbamem.2008.03.013 pmid: 18420026 |
| 20. |
Chang VT, Crispin M, Aricescu AR, et al. Glycoprotein structural genomics: solving the glycosylation problem. Structure,2007; 15(3):267-73. doi: 10.1016/j.str.2007.01.011.
doi: 10.1016/j.str.2007.01.011 pmid: 17355862 |
| 21. |
Elbein AD, Tropea JE, Mitchell M, et al. Kifunensine, a potent inhibitor of the glycoprotein processing mannosidase I. J Biol Chem,1990; 265(26):15599-605.
doi: 10.1016/0014-5793(90)81275-S pmid: 2144287 |
| 22. |
Flanagan JG, Leder P . The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell,1990; 63(1):185-94. doi: 10.1016/0092-8674(90)90299-T.
doi: 10.1016/0092-8674(90)90299-T pmid: 1698555 |
| 23. |
Flanagan JG, Cheng HJ . Alkaline phosphatase fusion proteins for molecular characterization and cloning of receptors and their ligands. Methods Enzymol,2000; 327:198-210. doi: 10.1016/S0076-6879(00)27277-7.
doi: 10.1016/S0076-6879(00)27277-7 |
| 24. |
Flanagan JG, Cheng HJ, Feldheim DA, et al. Alkaline phosphatase fusions of ligands or receptors as in situ probes for staining of cells, tissues, and embryos. Methods Enzymol,2000; 327:19-35. doi: 10.1016/S0076-6879(00)27264-9.
doi: 10.1016/S0076-6879(00)27264-9 |
| 25. |
Zhang Y, Chao T, Li R, et al. MicroRNA-128 inhibits glioma cells proliferation by targeting transcription factor E2F3a. J Mol Med (Berl),2009; 87(1):43-51. doi: 10.1007/s00109-008-0403-6.
doi: 10.1007/s00109-008-0403-6 pmid: 18810376 |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|