Chinese Medical Sciences Journal ›› 2018, Vol. 33 ›› Issue (2): 100-106.doi: 10.24920/11812
• Original Article • Previous Articles Next Articles
Cortical Thinning Pattern of Bulbar- and Spinal-onset Amyotrophic Lateral Sclerosis: a Surface-based Morphometry Study
Chen Zhiye1, Liu Mengqi1, Ma Lin2, *( )
)
- 1 Department of Radiology, Hainan Branch of Chinese PLA General Hospital, Sanya, hainan 572013, China
2 Department of Radiology, Chinese PLA General Hospital, Beijing 100853, China
-
Received:2017-07-17Published:2018-06-30Online:2018-05-28 -
Contact:Ma Lin E-mail:cjr.malin@vip.163.com
Cite this article
Chen Zhiye, Liu Mengqi, Ma Lin. Cortical Thinning Pattern of Bulbar- and Spinal-onset Amyotrophic Lateral Sclerosis: a Surface-based Morphometry Study[J].Chinese Medical Sciences Journal, 2018, 33(2): 100-106.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
"
| Cluster | Anatomic region | MNI-space | K value | Pvalue | tvalue | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| ALS vs. NC | |||||||
| 1 | Left precentral gyrus | -9 | -24 | 76 | 619 | 0.000 | 4.15 |
| 2 | Left postcentral gyrus | -56 | -9 | 19 | 293 | 0.000 | 3.70 |
| 3 | Right gyrus rectus | 4 | 43 | -22 | 150 | 0.000 | 3.41 |
| 4 | Right medial precentral gyrus | 6 | -22 | 73 | 191 | 0.000 | 3.37 |
| ALS-bulbar vs. NC | |||||||
| 5 | Left precentral gyrus | -25 | -16 | 68 | 154 | 0.000 | 4.20 |
| 6 | Righ SMC | 8 | -8 | 69 | 219 | 0.000 | 4.45 |
| ALS-spinal vs. NC | |||||||
| 7 | Left posterior insula | -36 | -13 | -5 | 264 | 0.000 | 3.85 |
| 8 | Right gyrus rectus | 5 | 42 | -24 | 210 | 0.000 | 3.51 |
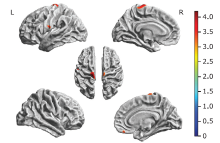
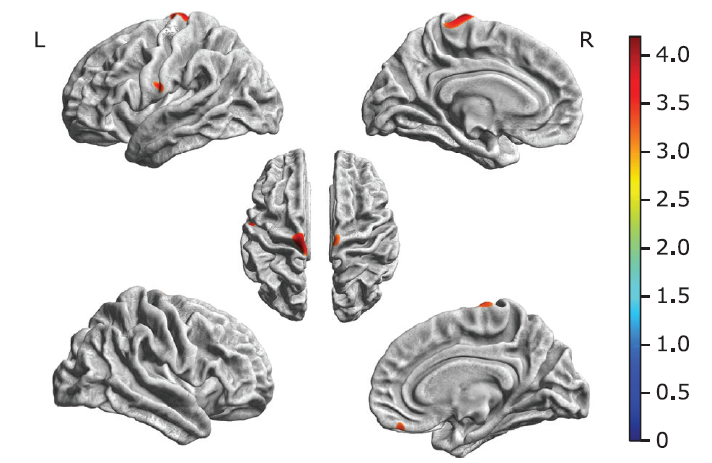
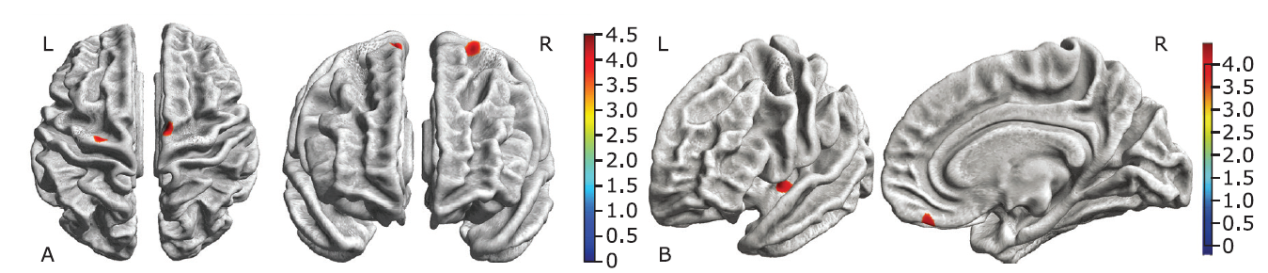
Figure 3.
Brain regions with decreased cortical thickness in the ALS-bulbar patients and in the ALS-spinal patients compared with the NC group. A. Decreased cortical thickness in ALS-bulbar located in the left precentral gyrus and the right supplementary motor cortex. B. Decreased cortical thickness in the ALS-spinal located in the left posterior insula and the right gyrus rectus. Color bar represent t value."
"
| Brain regions | Cortical thickness (mm) | Disease duration | ALSFRS-R score | |||
|---|---|---|---|---|---|---|
| r | Pvalue | r | Pvalue | |||
| ALS vs. NC | ||||||
| Left precentral gyrus | 2.77±0.22 | 0.003 | 0.979 | 0.245 | 0.053 | |
| Left postcentral gyrus | 2.26±0.19 | -0.052 | 0.684 | 0.126 | 0.326 | |
| Right gyrus rectus | 3.31±0.19 | -0.311 | 0.013 | -0.018 | 0.886 | |
| Right precentral gyrus | 2.88±0.15 | -0.081 | 0.528 | 0.271 | 0.032 | |
| ALS-spinal vs. NC | ||||||
| Left insula | 3.95±0.35 | -0.409 | 0.004 | -0.011 | 0.943 | |
| Right gyrus rectus | 3.34±0.19 | -0.351 | 0.014 | -0.022 | 0.881 | |
| ALS-bulbar vs. NC | ||||||
| Left precentral gyrus | 2.70±0.22 | -0.226 | 0.458 | 0.224 | 0.461 | |
| Right supplementary motor cortex | 2.19±0.14 | 0.071 | 0.818 | 0.204 | 0.505 | |
| 1. |
Chou SM, Norris FH . Amyotrophic lateral sclerosis: lower motor neuron disease spreading to upper motor neurons. Muscle Nerve 1993; 16(8):864-9. doi: 10.1002/mus.880160810.
doi: 10.1002/mus.880160810 |
| 2. |
van der Graaff MM, de Jong JM, Baas F , et al. Upper motor neuron and extra-motor neuron involvement in amyotrophic lateral sclerosis: a clinical and brain imaging review. Neuromuscul Disord 2009; 19(1):53-8. doi: 10.1016/j.nmd.2008.10.002.
doi: 10.1016/j.nmd.2008.10.002 pmid: 19070491 |
| 3. |
Pringle CE, Hudson AJ, Munoz DG , et al. Primary lateral sclerosis. Clinical features, neuropathology and diagnostic criteria. Brain 1992; 115(Pt2):495-520. doi: 10.1093/brain/115.2.495.
doi: 10.1093/brain/115.2.495 |
| 4. |
Pamphlett R, Kril J, Hng TM . Motor neuron disease: a primary disorder of corticomotoneurons? Muscle Nerve 1995; 18(3):314-8. doi: 10.1002/mus.880180308.
doi: 10.1002/mus.880180308 |
| 5. |
Abrahams S, Goldstein LH, Suckling J , et al. Frontotemporal white matter changes in amyotrophic lateral sclerosis. J Neurol 2005; 252(3):321-31. doi: 10.1007/s00415-005-0646-x.
doi: 10.1007/s00415-005-0646-x pmid: 15739047 |
| 6. |
Ashburner J, Friston KJ . Voxel-based morphometry—the methods. Neuroimage 2000; 11(6 Pt 1):805-21. doi: 10.1006/nimg.2000.0582.
doi: 10.1006/nimg.2000.0582 |
| 7. |
Thivard L, Pradat PF, Lehéricy S , et al. Diffusion tensor imaging and voxel based morphometry study in amyotrophic lateral sclerosis: relationships with motor disability. J Neurol Neurosurg Psychiatry 2007; 78(8):889-92. doi: 10.1136/jnnp.2006.101758.
doi: 10.1136/jnnp.2006.101758 pmid: 2117724 |
| 8. |
Grosskreutz J, Kaufmann J, Fr?drich J , et al. Widespread sensorimotor and frontal cortical atrophy in Amyotrophic Lateral Sclerosis. BMC Neurol 2006; 6(17):1-10. doi: 10.1186/1471-2377-6-17.
doi: 10.1186/1471-2377-6-17 |
| 9. |
Shen D, Cui L, Fang J , et al. Voxel-wise meta-analysis of gray matter changes in amyotrophic lateral sclerosis. Front Aging Neurosci 2016; 8(64):1-12. doi: 10.3389/fnagi.2016.00064.
doi: 10.3389/fnagi.2016.00064 pmid: 4811926 |
| 10. |
Sheng L, Ma H, Zhong J , et al. Motor and extra-motor gray matter atrophy in amyotrophic lateral sclerosis: quantitative meta-analyses of voxel-based morphometry studies. Neurobiol Aging 2015; 36(12):3288-99. doi: 10.1016/j.neurobiolaging.2015.08.018.
doi: 10.1016/j.neurobiolaging.2015.08.018 pmid: 26362941 |
| 11. |
Fornito A, Yücel M, Wood SJ , et al. Surface-based morphometry of the anterior cingulate cortex in first episode schizophrenia. Hum Brain Mapp 2008; 29(4):478-89. doi: 10.1002/hbm.20412.
doi: 10.1002/hbm.20412 |
| 12. |
Chen Z, Ma L . Grey matter volume changes over the whole brain in amyotrophic lateral sclerosis: A voxel-wise meta-analysis of voxel based morphometry studies. Amyotroph Lateral Scler 2010; 11(6):549-54. doi: 10.3109/17482968.2010.516265.
doi: 10.3109/17482968.2010.516265 pmid: 20929296 |
| 13. |
Kim H, Kim JH, Possin KL , et al. Surface-based morphometry reveals caudate subnuclear structural damage in patients with premotor Huntington disease. Brain Imaging Behav 2016; 11(5):1365-72. doi: 10.1007/s11682-016-9616-4.
doi: 10.1007/s11682-016-9616-4 pmid: 27730480 |
| 14. |
Huang P, Lou Y, Xuan M , et al. Cortical abnormalities in Parkinson’s disease patients and relationship to depression: A surface-based morphometry study. Psychiatry Res Neuroimaging 2016; 250:24-8. doi: 10.1016/j.pscychresns.2016.03.002.
doi: 10.1016/j.pscychresns.2016.03.002 pmid: 27107157 |
| 15. |
Lu H, Ma SL, Chan SS , et al. The effects of apolipoprotein epsilon 4 on aging brain in cognitively normal Chinese elderly: a surface-based morphometry study. Int Psychogeriatr 2016; 28(9):1503-11. doi: 10.1017/S1041610216000624.
doi: 10.1017/S1041610216000624 pmid: 27097839 |
| 16. | Butman JA, Floeter MK . Decreased thickness of primary motor cortex in primary lateral sclerosis. Am J Neuroradiol 2007; 28(1):87-91. |
| 17. |
Roccatagliata L, Bonzano L, Mancardi G , et al. Detection of motor cortex thinning and corticospinal tract involvement by quantitative MRI in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2009; 10(1):47-52. doi: 10.1080/17482960802267530.
doi: 10.1080/17482960802267530 pmid: 1862277218622772 |
| 18. |
Cosottini M, Donatelli G, Costagli M , et al. High-resolution 7T MR imaging of the motor cortex in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 2016; 37(3):455-61. doi: 10.3174/ajnr.A4562.
doi: 10.3174/ajnr.A4562 |
| 19. |
d’Ambrosio A, Gallo A, Trojsi F , et al. Frontotemporal cortical thinning in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 2014; 35(2):304-10. doi: 10.3174/ajnr.A3753.
doi: 10.3174/ajnr.A3753 pmid: 24113470 |
| 20. |
Chen Z, Liu M, Ma L . Gray matter volume changes over the whole brain in the bulbar- and spinal-onset amyotrophic lateral sclerosis: a voxel-based morphometry study. Chin Med Sci J 2018; 33(1):20-8. doi: 10.24920/11804.
doi: 10.24920/11804 pmid: 29620511 |
| 21. |
Brooks BR, Miller RG, Swash M , et al. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000; 1(5):293-9. doi: 10.1080/146608200300079536.
doi: 10.1080/146608200300079536 pmid: 11464847 |
| 22. |
Ohashi Y, Tashiro K, Itoyama Y , et al. Study of functional rating scale for amyotrophic lateral sclerosis: revised ALSFRS(ALSFRS-R) Japanese version. Brain and nerve 2001; 53(4):346-55. Japanese.
pmid: 11360474 |
| 23. |
Galea M, Woodward M . Mini-Mental State Examination (MMSE). Aust J Physiother 2005; 51(3):198. doi: 10.1016/S0004-9514(05)70034-9.
doi: 10.1016/S0004-9514(05)70034-9 |
| 24. |
Dahnke R, Yotter RA, Gaser C . Cortical thickness and central surface estimation. Neuroimage 2013; 65:336-48. doi: 10.1016/j.neuroimage.2012.09.050.
doi: 10.1016/j.neuroimage.2012.09.050 pmid: 23041529 |
| 25. |
Desikan RS, Ségonne F, Fischl B , et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31(3):968-80. doi: 10.1016/j.neuroimage.2006.01.021.
doi: 10.1016/j.neuroimage.2006.01.021 pmid: 16530430 |
| 26. |
Hamilton RL, Bowser R . Alzheimer disease pathology in amyotrophic lateral sclerosis. Acta Neuropathol 2004; 107(6):515-22. doi: 10.1007/s00401-004-0843-1.
doi: 10.1007/s00401-004-0843-1 pmid: 15024584 |
| 27. |
Chen Z, Ma L . Grey matter volume changes over the whole brain in amyotrophic lateral sclerosis: A voxel-wise meta-analysis of voxel based morphometry studies. Amyotroph Lateral Scler 2010; 11(6):549-54. doi: 10.3109/17482968.2010.516265.
doi: 10.3109/17482968.2010.516265 pmid: 20929296 |
| 28. |
Gredal O, Pakkenberg H, Karlsborg M , et al. Unchanged total number of neurons in motor cortex and neocortex in amyotrophic lateral sclerosis: a stereological study. J Neurosci Methods 2000; 95(2):171-6. doi: 10.1016/S0165-0270(99)00175-2.
doi: 10.1016/S0165-0270(99)00175-2 pmid: 10752488 |
| 29. |
Kiernan JA, Hudson AJ . Changes in shapes of surviving motor neurons in amyotrophic lateral sclerosis. Brain 1993; 116(Pt 1):203-15. doi: 10.1093/brain/116.1.203.
doi: 10.1093/brain/116.1.203 pmid: 8453457 |
| 30. |
Nagy D, Kato T, Kushner PD . Reactive astrocytes are widespread in the cortical gray matter of amyotrophic lateral sclerosis. J Neurosci Res 1994; 38(3):336-47. doi: 10.1002/jnr.490380312.
doi: 10.1002/jnr.490380312 pmid: 7523689 |
| [1] | Atefeh Beigi-khoozani, Amirmohammad Merajikhah, Mahdieh Soleimani. Magnetic Resonance Imaging Findings of Olfactory Bulb in Anosmic Patients with COVID-19: A Systematic Review [J]. Chinese Medical Sciences Journal, 2022, 37(1): 23-30. |
| [2] | Jia Xu, Xuan Wang, Zhengyu Jin, Qin Wang, Yan You, Shitian Wang, Tianyi Qian, Huadan Xue. Assessing Liver Function by T1 Maps on Gd-EOB-DTPA-Enhanced MRI for up to 50 Min in Rat Models of Liver Fibrosis: A Longer Hepatobiliary Time Period may Help [J]. Chinese Medical Sciences Journal, 2021, 36(2): 110-119. |
| [3] | Wang Xuedan, Wang Shiwei, Wang Botao, Chen Zhiye. Effect of MR Field Strength on the Texture Features of Cerebral T2-FLAIR Images: A Pilot Study [J]. Chinese Medical Sciences Journal, 2020, 35(3): 248-253. |
| [4] | Xu Yanhong,Yang Jia,Meng Jie,Wang Han. Targeted MR Imaging Adopting T1-Weighted Ultra-Small Iron Oxide Nanoparticles for Early Hepatocellular Carcinoma: An in vitro and in vivo Study [J]. Chinese Medical Sciences Journal, 2020, 35(2): 142-150. |
| [5] | Xu Jia, Wang Xuan, Jin Zhengyu, You Yan, Wang Qin, Wang Shitian, Xue Huadan. Value of Texture Analysis on Gadoxetic Acid-enhanced MR for Detecting Liver Fibrosis in a Rat Model [J]. Chinese Medical Sciences Journal, 2019, 34(1): 24-32. |
| [6] | Wang Botao, Fan Wenping, Xu Huan, Li Lihui, Zhang Xiaohuan, Wang Kun, Liu Mengqi, You Junhao, Chen Zhiye. Value of Magnetic Resonance Imaging Texture Analysis in the Differential Diagnosis of Benign and Malignant Breast Tumors [J]. Chinese Medical Sciences Journal, 2019, 34(1): 33-37. |
| [7] | Li Ping, Zhu Liang, Wang Xuan, Xue Huadan, Wu Xin, Jin Zhengyu. Imaging Diagnosis of Type Ⅲ Choledochal Cyst: A Case Report [J]. Chinese Medical Sciences Journal, 2018, 33(3): 194-203. |
| [8] | Li Lihui, Huang Houbin, Chen Zhiye. Early Diagnosis of Recurrent Optic Neuritis Using Contrast-Enhanced T2 Fluid-attenuated Inversion Recovery Imaging: a Case Report [J]. Chinese Medical Sciences Journal, 2018, 33(2): 130-134. |
| [9] | Chen Zhiye,Liu Mengqi,Ma Lin. Gray Matter Volume Changes over the Whole Brain in the Bulbar- and Spinal-onset Amyotrophic Lateral Sclerosis: a Voxel-based Morphometry Study [J]. Chinese Medical Sciences Journal, 2018, 33(1): 20-28. |
| [10] | Liu Mengqi, Chen Zhiye, Ma Lin. Reliability of Three Dimentional Pseudo-continuous Arterial Spin Labeling: A Volumetric Cerebral Perfusion Imaging with Different Post-labeling Time and Functional State in Health Adults [J]. Chinese Medical Sciences Journal, 2018, 33(1): 38-44. |
| [11] | Pan Haipeng, Lao Qun, Fei Zhenghua, Yang Li, Zhou Haichun, Lai Can. MR Lymphangiography for Focal Disruption of the Thoracic Duct in Chylothorax of an Infant: a Case Report and Literature Review△ [J]. Chinese Medical Sciences Journal, 2017, 32(4): 265-268. |
| [12] | Chen Zhiye, Zang Xiujuan, Liu Mengqi, Liu Mengyu, Li Jinfeng, Gu Zhaoyan, Ma Lin. Abnormal Alterations of Cortical Thickness in 16 Patients with Type 2 Diabetes Mellitus: A Pilot MRI Study△ [J]. Chinese Medical Sciences Journal, 2017, 32(2): 75-82. |
| [13] | Wang Ting, Ma Lin, Lou Xin, Bu Bo. Trigeminal Ganglioneuroma in the Middle-posterior Cranial Fossa: a Case Report△ [J]. Chinese Medical Sciences Journal, 2017, 32(2): 123-128. |
| [14] | Yu Wang, Zi-yuan Liu, Wan-chen Dou, Wen-bin Ma, Ren-zhi Wang, Yi Guo. Application of Preoperative CT/MRI Image Fusion in Target Positioning for Deep Brain Stimulation [J]. Chinese Medical Sciences Journal, 2016, 31(3): 161-167. |
| [15] | Xiang Quan, Tie-hu Ye, Si-fang Lin, Liang Zou, Shou-yuan Tian. Propofol Affects Different Human Brain Regions Depending on Depth of Sedation [J]. Chinese Medical Sciences Journal, 2015, 30(3): 135-142. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|