Chinese Medical Sciences Journal ›› 2017, Vol. 32 ›› Issue (1): 1-12.doi: 10.24920/J1001-9242.2007.001
• Orginal Article • Next Articles
Efficacy of Short-term Dual Antiplatelet Therapy after Implantation of Second-generation Drug-eluting Stents: A Meta-analysis and Systematic Review
Huang Peisen1#, Yu Yuan1#, Han Xikun1, Yang Yuejin1, *( )
)
- 1State Key Laboratory of Cardiovascular Disease
2National Clinical Research Center of Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100037, China
-
Published:2017-03-31Online:2017-04-10 -
Contact:Yang Yuejin E-mail:yangyjfw@126.com
Cite this article
Huang Peisen, Yu Yuan, Han Xikun, Yang Yuejin. Efficacy of Short-term Dual Antiplatelet Therapy after Implantation of Second-generation Drug-eluting Stents: A Meta-analysis and Systematic Review[J].Chinese Medical Sciences Journal, 2017, 32(1): 1-12.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 1
Results of bias risk assessment of the 5 included studies"
| Methodological items | ITALIC12 | OPTIMIZE14 | RPRODIGY15 | RESET16 | SECURITY13 |
|---|---|---|---|---|---|
| Random sequence generation | Yes (Web-based) | Yes | Yes (Computer- generated) | Yes (Web-based) | Yes |
| Allocation concealment | Yes (Centralized randomization) | Unclear | Yes (Sealed envelopes) | Unclear | Unclear |
| Blinding of participants* | Yes | Yes | Yes | Yes | Yes |
| Blinding of outcome assessment | Yes | Yes | Yes | Yes | Yes |
| Incomplete outcome data | Yes | Yes | Yes | Yes | Yes |
| Selective outcome reporting | Yes | Yes | Yes | Yes | Yes |
| Sample size calculation | Yes | Yes (Non-inferiority design) | Yes | Yes (Non-inferiority design) | Yes (Non inferiority design) |
| Funding sources disclosure | Yes (Industry-funded) | Yes (Industry-funded) | Yes (Investigator- driven) | Yes (Public Health- and Industry-funded ) | Yes (Public Health-funded ) |
| Other bias | Yes (Prematurely terminative) | No | No | No | Yes (Prematurely terminative) |
Table 2
Main characteristics of the 5 included trials"
| Items | ITALIC12 | SECURITY13 | OPTIMIZE14 | PRODIGY15* | RESET16 |
|---|---|---|---|---|---|
| Patients (n) | 1850 | 1399 | 3119 | 988 | 2117 |
| Age (yrs) | 61.6 | 65.2 | 61.6 | 67.7 | 62.4 |
| Male (%) | 80 | 77.2 | 63.3 | 78.7 | 63.6 |
| BMI (kg/m2) | 27 | NA | NA | 27.5 | 25 |
| Current smoker (%) | 51.8 | 22.2 | 17.9 | 23.9 | 24 |
| Previous PCI (%) | 23.3 | 17.7 | 20.0 | NA | 3.3 |
| Comorbidities | |||||
| Diabetes (%) | 37.0 | 30.7 | 35.4 | 23.3 | 29.3 |
| Dyslipidaemia (%) | 67.1 | 63 | 61.1 | 60.0 | 58.8 |
| Hypertension (%) | 65.0 | 72.8 | 87.2 | 69.6 | 61.9 |
| Severity of illness | |||||
| Ejection fraction (%) | NA | 56.5 | NA | 50.8 | 64.1 |
| Silent ischemia (%) | 20.2 | - | 8.9 | - | - |
| Stable CAD (%) | 41.3 | 50.7 | 59.2 | 25.2 | 45.4 |
| ACS (%) | 23.5 | 31.6 | 31.9 | 74.8 | 54.6 |
| Procedural characteristics | |||||
| Multivessel disease (%) | NA | 42.3 | 25.9 | 71.4 | 22.7 |
| LAD treated (%) | 72.8 | 43.5 | 47.2 | 58.9 | 53.2 |
| Lesion length (mm) | NA | 19 | 18.4 | 13.45 | 19.8 |
| Stent/lesion | NA | NA | 1.2 | 1.2 | 1.0 |
| Type of DES used | EES | ZES, EES | ZES | ZES, EES | EES, SES, ZES |
| Thienopyridine category | Clopidogrel (98.2%), prasugrel and ticagrelor (1.8%) | Clopidogrel (99.9%), prasugrel and ticagrelor (0.1%) | Clopidogrel (100%) | clopidogrel (100%) | Clopidogrel (100%) |
| Main inclusion criteria | ≥18 years; PCI suitability; Xience V DES implantation; all clinical situations excluding primary PCI for acute MI and treatment of the left main artery | Stable angina or unstable angina or patients with documented silent ischemia; second-generation DES implantation <24 hours; ≥1 coronary artery lesion; ≥70% DS; ≥18 years, no other DES implantation; no BMS implantation ≤3 months | Stable angina, silent ischemia, unstable angina, recent (but not acute) MI (<30 days); ≥1 lesion; ≥50% DS; RVD ≥2.5 mm; PCI suitablity | ≥18 years; ≥1 coronary artery lesion; ≥50% DS; PCI suitability; RVD ≥2.25 mm; chronic stable coronary artery disease or ACS (NSTEMI/ STEMI) | ≥20 years; ≥50% DS; RVD 2.5 to 4.0 mm; elective PCI; stable angina, unstable angina, or acute MI |
| Items | ITALIC12 | SECURITY13 | OPTIMIZE14 | PRODIGY15* | RESET16 |
| Main exclusion criteria | Aspirin resistance; prior DES implantation <1 year; bleeding diathesis or active bleeding; DAPT contraindications; major surgery <6 weeks; severe liver failure; elective surgery planned <1 year; life expectancy <2 years | Patients treated for saphenous vein graft and in-stent restenosis; <48 hours STEMI; <6 months NSTEMI; LVEF <30%; DAPT contraindications; creatinine >177 μmol/L; bleeding diathesis or active bleeding; life expectancy <2 years | STEMI presenting for primary or rescue PCI; BMS implantation <6 months; previous treatment with any DES; elective surgery planned <12 months; DAPT contraindications; saphenous vein graft lesion | Elective surgery planned <24 months after index PCI (unless DAPT could be maintained thro- ughout the peri-surgical period); bleeding diathesis; major surgery <15 days; active bleeding or previous stroke <6 months; concomitant or foreseeable need for anticoagulants | Cerebral/peripheral atherosclerotic arterial disease, thromboembolic disease or ST history; LVEF <40%; restenotic lesion; CTO; LM disease requi- ring intervention; cardiogenic shock; STEMI<48 hours |
| Primary endpoint | Composite of all-cause death, MI, repeat emergency TVR, stroke, or major bleeding | Composite of cardiac death, MI, stroke, ST, or BARC type 3 or 5 bleeding | NACCE (composite of all-cause death, MI, stroke, or major bleeding) | Composite of all-cause death, MI or CVAs | Composite of cardiac death, MI, ST, ID-TVR and TIMI major or minor bleeding |
| Secondary endpoint | All-cause death, MI, repeat emergency TVR, stroke; minor and minimal bleeding | Cardiac death, MI, stroke, ST, or BARC type 2, 3, or 5 bleeding; CI of the individual components of the primary endpoint; ID-TVR; all-cause mortality | ST; ID-TVR; MACE (composite of all-cause death, MI and any revascularization); bleeding | All-cause death; MI; CVAs; cardiac death; ST | Composite of all-cause death; MI; ST |
| Time to randomization | 6 months after index PCI | At index PCI | At index PCI | 1 month after index PCI | At index PCI |
| DAPT duration(mon) | |||||
| Short-term DAPT group | 6 | 6 | 3 | 6 | 3 |
| Standard DAPT group | 12 | 12 | 12 | 24 | 12 |
| Year of publication | 2015 | 2014 | 2013 | 2013 | 2012 |
| Registration number | NCT01476020 | NCT00944333 | NCT01113372 | NCT00611286 | NCT01145079 |

Figure 2.
Comparison of total efficacy of short-term dual antiplatelet therapy (DAPT) vs. standard DAPT. The squares and the horizontal lines indicate the risk ratio and the (95% CI) for each trial included; the size of each square is proportional to the statistical weight of a trial in the meta-analysis; diamond indicates the effect estimate derived from meta-analysis, with the center indicating the point estimate and the left and the right ends of the (95% CI)."
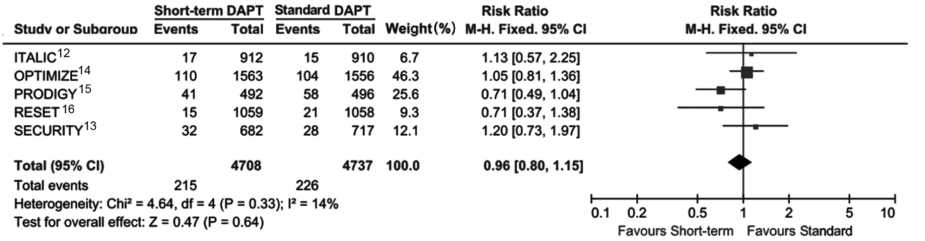
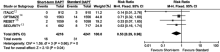
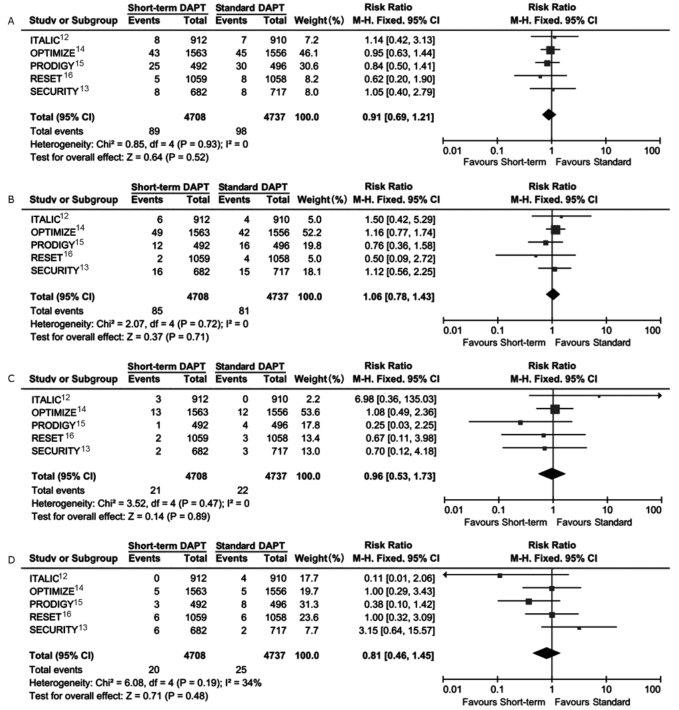
Figure 4.
Comparison of major bleeding risk of patients receiving short-term DAPT vs. standard DAPT. The squares and the horizontal lines indicate the risk ratio and the (95% CI) for each trial included; the size of each square is proportional to the statistical weight of a trial in the meta-analysis; diamond indicates the effect estimate derived from meta-analysis, with the center indicating the point estimate and the left and the right ends of the (95% CI)."


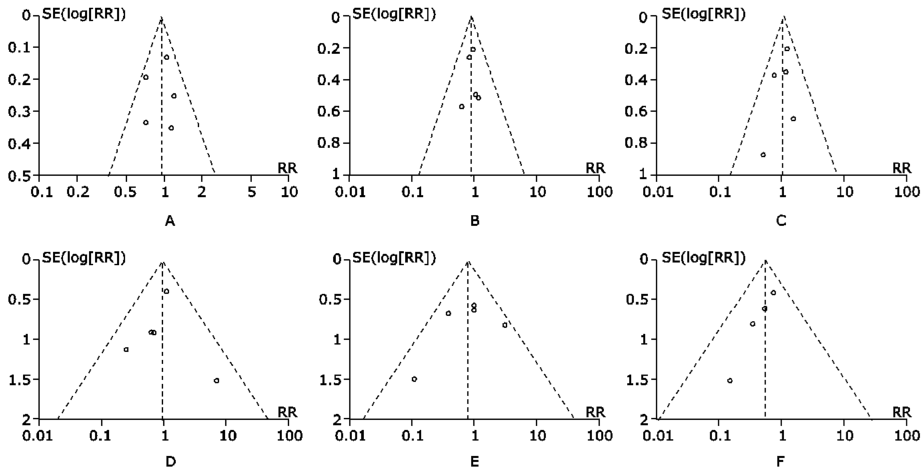
Figure 6.
Sensitivity analysis of all studies assessing association of difference in DAPT duration between study arms on meta- analysis results for overall efficacy endpoint and individual endpoints.A. Meta-regression for efficacy effect; B. all-cause death; C. myocardial infarction; D. definite or probable stent thrombosis; E. cerebrovascular accidents. RR: relative risk."


Figure 7.
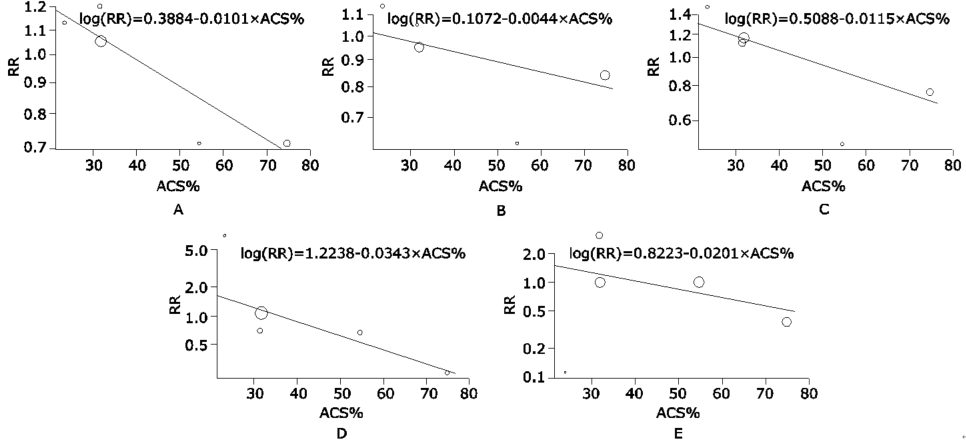
Sensitivity analysis of all studies assessing association of proportion of patients with acute coronary syndrome (ACS%) between study arms on meta-analysis results for overall efficacy endpoint and individual endpoints.A. Meta-regression for efficacy effect; B. all-cause death; C. myocardial infarction; D. definite or probable stent thrombosis; E. cerebrovascular accidents."
| 1. | Mauri L, Kereiakes DJ, Yeh RW, et al.Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371:2155-66. |
| 2. | Wijns W, Steg PG, Mauri L, et al.Endeavour zotarolimus-eluting stent reduces stent thrombosis and improves clinical outcomes compared with cypher sirolimus-eluting stent: 4-year results of the PROTECT randomized trial. Eur Heart J 2014; 35:2812-20. |
| 3. | Dangas GD, Serruys PW, Kereiakes DJ, et al.Meta-analysis of everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease: final 3-year results of the SPIRIT clinical trials program (Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv 2013; 6:914-22. |
| 4. | Finn AV, Joner M, Nakazawa G, et al.Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 2007; 115:2435-41. |
| 5. | Kandzari DE, Barker CS, Leon MB, et al.Dual antiplatelet therapy duration and clinical outcomes following treatment with zotarolimus-eluting stents. JACC Cardiovasc Interv 2011; 4:1119-28. |
| 6. | Windecker S, Kolh P, Alfonso F, et al.2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014; 35:2541-619. |
| 7. | Levine GN, Bates ER, Blankenship JC, et al.2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44-e122. |
| 8. | Ziada KM, Abdel-Latif A, Charnigo R, et al.Safety of an abbreviated duration of dual antiplatelet therapy (≤6 months) following second-generation drug-eluting stents for coronary artery disease: a systematic review and meta-analysis of randomized trials. Catheter Cardiovasc Interv 2016; 87:722-32. |
| 9. | Higgins JPT, Sterne JAC, Altman DG, et al.The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:889-93. |
| 10. | Higgins JP, Thompson SG, Deeks JJ, et al.Measuring inconsistency in meta-analyses. BMJ 2003; 327:557-60. |
| 11. | Sterne JA, Egger M, Smith GD.Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ 2001; 323: 101-5. |
| 12. | Gilard M, Barragan P, Noryani AA, et al.6- versus 24-month dual antiplatelet therapy after implantation of drug-eluting stents in patients nonresistant to aspirin: the randomized, multicenter ITALIC trial. J Am Coll Cardiol 2015; 65:777-86. |
| 13. | Colombo A, Chieffo A, Frasheri A, et al.Second-generation drug-eluting stent implantation followed by 6-versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014; 64: 2086-97. |
| 14. | Feres F, Costa RA, Abizaid A, et al.Three vs. twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013; 310:2510-22. |
| 15. | Valgimigli M, Borghesi M, Tebaldi M, et al.Should duration of dual antiplatelet therapy depend on the type and/ or potency of implanted stent? A pre-specified analysis from the PROlonging dual antiplatelet treatment after grading stent-induced intimal hyperplasia study (PRODIGY). Eur Heart J 2013; 34:909-19. |
| 16. | Kim BK, Hong MK, Shin DH, et al.A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (real safety and efficacy of 3-month dual antiplatelet therapy following endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012; 60:1340-8. |
| 17. | Chen ZM, Jiang LX, Chen YP, et al.Addition of clopidogrel to aspirin in 45 852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607-21. |
| 18. | Sardella G, Mancone M, Biondi-Zoccai G, et al.Beneficial impact of prolonged dual antiplatelet therapy after drug-eluting stent implantation. J Interv Cardiol 2012; 25:596-603. |
| 19. | Faxon DP, Lawler E, Young M, et al.Prolonged clopidogrel use after bare metal and drug-eluting stent placement: the veterans administration drug-eluting stent study. Circ Cardiovasc Interv 2012; 5:372-80. |
| 20. | Eisenstein EL, Kandzari DE, Peterson ED, et al.Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA 2007; 297:159-68. |
| 21 | 21.Tanzilli G, Greco C, Pelliccia F, et al. Effectiveness of two-year clopidogrel + aspirin in abolishing the risk of very late thrombosis after drug-eluting stent implantation (from the TYCOON [two-year ClOpidOgrel need] study). Am J Cardiol 2009; 104:1357-61. |
| 22. | Kwok CS, Bulluck H, Ryding AD, et al.Benefits and harms of extending the duration of dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stents: a meta-analysis. Scientific World J 2014; 2014: 794078. |
| 23. | Palmerini T, Benedetto U, Bacchi-Reggiani L, et al.Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet 2015; 385:2371-82. |
| 24. | Spencer FA, Prasad M, Vandvik PO, et al.Longer- versus shorter-duration dual-antiplatelet therapy after drug-eluting stent placement: a systematic review and meta-analysis. Ann Intern Med 2015; 163:118-26. |
| 25. | Giustino G, Baber U, Sartori S, et al.Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol 2015; 65:1298-310. |
| 26. | Yeh RW, Mauri L, Kereiakes DJ.Dual antiplatelet therapy duration following coronary stenting. J Am Coll Cardiol 2015; 65:787-90. |
| 27. | Bonaca MP, Bhatt DL, Cohen M, et al.Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372:1791-800. |
| 28. | Montalescot G, Wiviott SD, Braunwald E, et al.Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet 2009; 373:723-31. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|