Chinese Medical Sciences Journal ›› 2018, Vol. 33 ›› Issue (1): 1-8.doi: 10.24920/11805
• 论著 • 下一篇
鼠源Nectin-like 4糖蛋白在293ET细胞系中的重组表达与纯化
- 中国医学科学院基础医学研究所,北京协和医学院基础学院,国家医学分子生物重点实验室,北京 100005
-
收稿日期:2017-10-23出版日期:2017-03-17发布日期:2018-03-07
Recombinant Expression and Purification of Mouse Nectin-like 4 Glycoprotein in 293ET Cell Line
Li Dongdong,An Tai,Liu Xiao,Yin Bin,Peng Xiaozhong( ),Shu Pengcheng(
),Shu Pengcheng( )
)
- State Key Laboratory of Medical Molecular Biology, Institute of Basic Medical Sciences Chinese Academy of Medical Sciences & School of Basic Medicine Peking Union Medical College, Beijing 100005, China
-
Received:2017-10-23Published:2017-03-17Online:2018-03-07 -
About author:Corresponding author Tel: 86-10-69156434, Fax: 86-10-65240529; E-mail: Xiaozhong Pengpengxiaozhong@pumc.edu.cn |Corresponding author Tel: 86-10-69156434, Fax: 86-10-65240529; E-mail: Pengcheng Shupengcheng_shu@ibms.pumc.edu.cn
摘要: 目的 筛选可以高水平表达Nectin-like 4 蛋白的稳定细胞系。方法 首先,将Necls胞外区的cDNA序列插入到修饰载体pAPtag5上碱性磷酸酶(AP)的N端,使其融合表达。其次,在293ET细胞中分泌表达Necls-AP融合蛋白,并通过AP活性和蛋白质免疫印迹等方法检测蛋白表达情况。然后,通过在细胞培养过程中加入N-糖链形成抑制剂以抑制复杂糖链的形成。在纯化的蛋白溶液中加入糖苷酶去除剩余的糖链。最后,使用人鼻病毒3C蛋白酶将融合蛋白上的AP蛋白切除,通过凝胶排阻色谱将该蛋白与目的蛋白分离。最终获得的目的蛋白在280nm波长下检测获得其浓度,通过SDS-PAGE电泳结果检测分析蛋白纯度。结果 通过利用重组载体上AP蛋白标签的颜色反应,我们筛选得到高水平表达Necl-4蛋白的稳转细胞系。在对目的蛋白进行了去糖基化等处理,我们最终可在1L的细胞培养上清中纯化获得4mg可溶、有活性的Necl-4蛋白,且蛋白纯度在95%左右。结论 使用AP蛋白—哺乳动物细胞表达系统,通过对AP蛋白的活性分析,我们快速筛选得到蛋白高表达量的稳转细胞系。在细胞培养中添加糖链抑制剂以及用糖苷酶处理纯化后的蛋白,可以得到了毫克级的去糖基化蛋白Necl-4。.本文中所使用的方法,对于表达、纯化得到糖蛋白用于进一步的结构和功能研究提供了思路。
引用本文
Li Dongdong, An Tai, Liu Xiao, Yin Bin, Peng Xiaozhong, Shu Pengcheng. Recombinant Expression and Purification of Mouse Nectin-like 4 Glycoprotein in 293ET Cell Line[J].Chinese Medical Sciences Journal, 2018, 33(1): 1-8.
Table 1
Primers used for Necl extracellular region cloning"
| Genes | 5’ primer | 3’ primer |
|---|---|---|
| mNecl-1 | 5’-CCCAAGCTTACATCTTTCCCAGGACGATAG-3’ | 5’-GAAGATCTGTGGTAGGTACTGGAGGAC-3’ |
| mNecl-2 | 5’-CCCAAGCTTACCAGAATCTGTTTACTAAAGACGTG-3’ | 5’-GAAGATCTGTGGTCCACTGCCCC-3’ |
| mNecl-3 | 5’-GAAGATCTCAGTTTCCACTAACTCAGAATG-3’ | 5’-GAAGATCTATGGTCAGGGCCATTCTG-3’ |
| mNecl-4 | 5’-CCCAAGCTTACCAGGAAGTACAGACCGAG-3’ | 5’-GAAGATCTGGCGTAAGGAACCGATG-3’ |

Figure 1.
Schematic disgram of pAPtag5-TEV (A) and pAPtag5-3C (B) modified from mammalian expression vector pAPtag5. The vector contains the Igκ chain singal peptide, followed by three enzyme restriction sites indicated in italicisized that are available for cloning. SP: signal peptide; PCMV: cytomegalovirus immediate/early promoter and enhancer element; TEV: tobacco etch potyvirus coding sequence; 3C: rhinovirus 3C protease coding sequence; AP: alkaline phosphatase coding sequence; SV40: SV40 DNA replication."
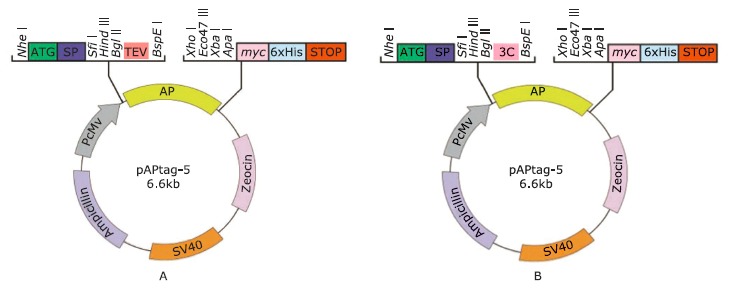
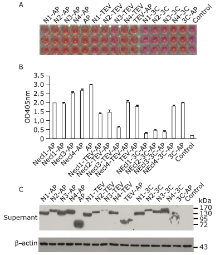
Figure 2.
Fast and easy screening the expression level of Necl-AP fusion protein with pAPtag5, pAPtag5-TEV or pAPtag5-3C recombinant vectors in 293ET cells. A. Color reaction results of the pNPP and AP fusion protein. Orange color means high level expression, pink color means low level expression. B. The column chart above shows the results of absorbance of Necls-AP fusion protein at 405 nm. Each reaction is repeated three times. C. Immunoblot analysis of protein expression in 293ET cells transduced with pAPtag5-Necls, pAPtag5-TEV-Necls or pAPtag5-3C-Necls recombinant vectors. Necl: Nectin-like protein; AP: alkaline phosphatase; pNPP: para-nitrophenylphosphate."
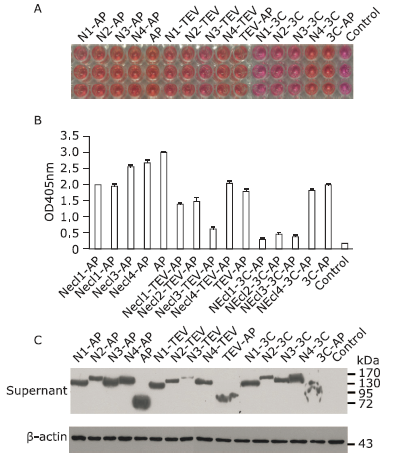
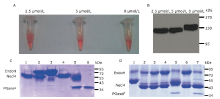
Figure 3.
Endo H and PGaseF digestion of Necl-4 produced in 293ET cells before and after adding kifunensine under various conditions. A. Color reaction results of pNPP and Necl4-AP fusion protein secreted from Necl4-AP/293ET stable cell line subjected to different concentrations of kifunensine. B. Immunoblot analysis of protein expression in Necl4-AP/293ET stable cell line cultured with kifunensine. C. Endo H and PGaseF digested Necl-4 without adding kifunensine in the culture medium. Lane 1: purified Necl-4 protein; Lane 2: Necl-4 cut by 1 kU Endo H after 6 hours; Lane 3: Endo H protein; Lane 4: purified Necl-4 protein; Lane 5: Necl-4 cut by 1 kU PGaseF after 6 hours; Lane 6: PGaseF protein. D. Endo H and PGaseF digested purified Necl-4 by adding kifunensine in the culture medium. Lane 1-5 purified Necl-4 protein was digested in non-reduced state. Lane 1: Necl-4 protein; Lane 2-4: Necl-4 cut by 0.25, 0.5 and 1 kU Endo H after 1 hour; Lane 5: Necl-4 cut by 0.5 kU PGaseF after 1 hour. Lane 6-7 Necl-4 protein was digested in reduced state. Lane 6: Necl-4 cut by 1 kU Endo H after 6 hours; Lane 7: Necl-4 cut by 0.5 kU PGaseF after 6 hours. Molecular weight of protein (Endo H: 80 kDa; Necl-4: 55 kDa; PGaseF: 36 kDa). Endo H: endoglycosidase H. PNGaseF: peptide N-glycosidase."
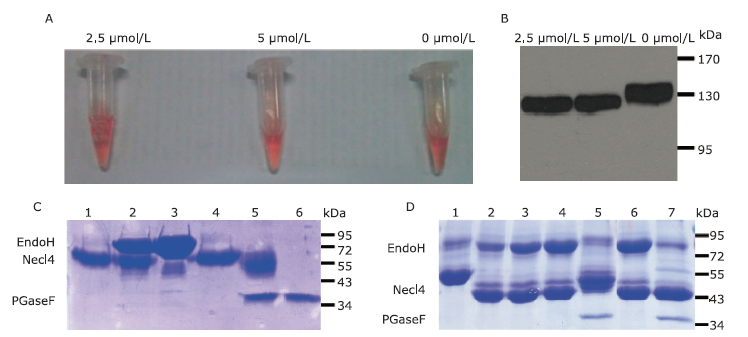
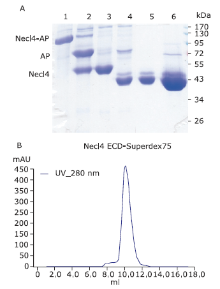
Figure 4.
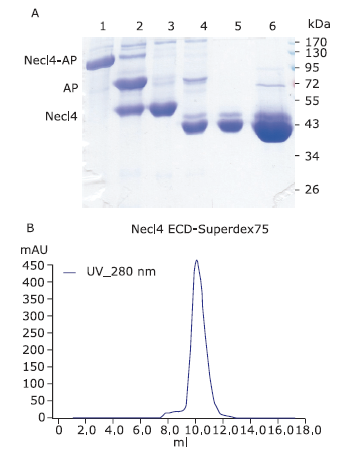
The large-scale purification process of deglycosylated Necl4 protein. A. Purification process of mouse Necl-4 protein in 293ET cell line by using SDS-PAGE analysis with Coomassie blue staining. Lane 1: purifed Necl4-AP fusion protein from cell supernant when clone was treated with 5 μmol/L kifunensine; Lane 2: after cleavage with 3C protease; Lane 3: after 3C protease and AP-tag were removed; Lane 4: after glycan chain of Necl-4 cut with Endo H enzyme; Lane 5: the purified Necl-4 after amylose resin; Lane 6: the concentrated Necl-4 protein after dialysis. Molecular weight of protein (Necl4-AP fusion protein: 130 kDa; AP: 80 kDa; Necl-4 before cutting glycan chain: 50 kDa; Necl-4 after cutting glycan chain: 43 kDa). B. Necl-4 extracellular coding domain purified by superdex 75 gel filtration column connected to an ?KTA FPLC purifier. The main panel shows the elution profile of Necl-4 extracellular coding domain from the column. The X-coordinate shows the elution volume of protein, the Y-coordinate shows the absorb of protein at 280 nm."
| 1. |
Samanta D, Almo SC . Nectin family of cell-adhesion molecules: structural and molecular aspects of function and specificity. Cell Mol Life Sci,2015; 72(4):645-58. doi: 10.1007/s00018-014-1763-4.
doi: 10.1007/s00018-014-1763-4 pmid: 25326769 |
| 2. |
Mandai K, Rikitake Y, Mori M, et al. Nectins and nectin-like molecules in development and disease. Curr Top Dev Biol,2015; 112:197-231. doi: 10.1016/bs.ctdb.2014.11.019.
doi: 10.1016/bs.ctdb.2014.11.019 |
| 3. |
Galibert L, Diemer GS, Liu Z, et al. Nectin-like protein 2 defines a subset of T-cell zone dendritic cells and is a ligand for class-I-restricted T-cell-associated molecule. J Biol Chem,2005; 280(23):21955-64. doi: 10.1074/jbc.M502095200.
doi: 10.1074/jbc.M502095200 |
| 4. |
Kakunaga S, Ikeda W, Itoh S, et al. Nectin-like molecule-1/TSLL1/SynCAM3: a neural tissue-specific immunoglobulin-like cell-cell adhesion molecule localizing at non-junctional contact sites of presynaptic nerve terminals, axons and glia cell processes. J Cell Sci,2005; 118(6):1267-77. doi: 10.1242/jcs.01656.
doi: 10.1242/jcs.01656 |
| 5. |
Mizutani K, Takai Y . Nectin spot: a novel type of nectin-mediated cell adhesion apparatus. Biochem J,2016; 473(18):2691-715. doi: 10.1042/BCJ20160235.
doi: 10.1042/BCJ20160235 pmid: 27621480 |
| 6. |
Catros V, Dessarthe B . Nectins and nectin-like recaeptors DNAM-1 and CRTAM: new ways for tumor escape. Med Sci,2014; 30(5):537-43. doi: 10.1051/medsci/20143005017
doi: 10.1051/medsci/20143005017 pmid: 24939541 |
| 7. |
Zhou Y, Du G, Hu X, et al. Nectin-like molecule 1 is a protein 4.1N associated protein and recruits protein 4.1N from cytoplasm to the plasma membrane. Biochim Biophys Acta,2005; 1669(2):142-54. doi: 10.1016/j.bbamem.2005.01.013.
doi: 10.1016/j.bbamem.2005.01.013 pmid: 15893517 |
| 8. |
Maurel P, Einheber S, Galinska J, et al. Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination. J Cell Biol,2007; 178(5):861-74. doi: 10.1083/jcb.200705132.
doi: 10.1083/jcb.200705132 pmid: 17724124 |
| 9. |
Spiegel I, Adamsky K, Eshed Y, et al. A central role for Necl4 (SynCAM4) in Schwann cell-axon interaction and myelination. Nat Neurosci,2007; 10(7):861-9. doi: 10.1038/nn1915.
doi: 10.1038/nn1915 pmid: 17558405 |
| 10. |
Park J, Liu B, Chen T, et al. Disruption of Nectin-like 1 cell adhesion molecule leads to delayed axonal myelination in the CNS. J Neurosci,2008; 28(47):12815-9. doi: 10.1523/JNEUROSCI.2665-08.2008.
doi: 10.1523/JNEUROSCI.2665-08.2008 pmid: 19036974 |
| 11. |
Heffernan C, Jain MR, Liu T, et al. Nectin-like 4 complexes with choline transporter-like protein-1 and regulates Schwann cell choline homeostasis and lipid biogenesis in vitro. J Biol Chem,2017; 292(11):4484-98. doi: 10.1074/jbc.M116.747816.
doi: 10.1074/jbc.M116.747816 pmid: 28119456 |
| 12. |
Zhu Y, Li H, Li K, et al. Necl-4/SynCAM-4 is expressed in myelinating oligodendrocytes but not required for axonal myelination. PLoS One,2013; 8(5):e64264. doi: 10.1371/journal.pone.0064264.
doi: 10.1371/journal.pone.0064264 |
| 13. |
Golan N, Kartvelishvily E, Spiegel I, et al. Genetic deletion of Cadm4 results in myelin abnormalities resembling Charcot-Marie-Tooth neuropathy. J Neurosci,2013; 33(27):10950-61. doi: 10.1523/JNEUROSCI.0571-13.2013.
doi: 10.1523/JNEUROSCI.0571-13.2013 |
| 14. |
Yang S, Weng H, Chen L, et al. Lack of protein 4.1G causes altered expression and localization of the cell adhesion molecule nectin-like 4 in testis and can cause male infertility. Mol Cell Biol,2011; 31(11): 2276-86. doi: 10.1128/MCB.01105-10.
doi: 10.1128/MCB.01105-10 |
| 15. |
Raveh S, Gavert N, Spiegel I, et al. The cell adhesion nectin-like molecules (Necl) 1 and 4 suppress the growth and tumorigenic ability of colon cancer cells. J Cell Biochem,2009; 108(1):326-36. doi: 10.1002/jcb.22258.
doi: 10.1002/jcb.22258 |
| 16. |
Dong X, Xu F, Gong Y, et al. Crystal structure of the V domain of human Nectin-like molecule-1/Syncam3/Tsll1/Igsf4b, a neural tissue-specific immunoglobulin-like cell-cell adhesion molecule. J Biol Chem,2006; 281(15):10610-7. doi: 10.1074/jbc.M513459200.
doi: 10.1074/jbc.M513459200 |
| 17. |
Fogel AI, Li Y, Giza J, et al. N-glycosylation at the SynCAM (synaptic cell adhesion molecule) immunoglobulin interface modulates synaptic adhesion. J Biol Chem,2010; 285(45):34864-74. doi: 10.1074/jbc.M110.120865.
doi: 10.1074/jbc.M110.120865 |
| 18. |
Liu J, Qian X, Chen Z, et al. Crystal structure of cell adhesion molecule nectin-2/CD112 and its binding to immune receptor DNAM-1/CD226. J Immunol,2012; 188(11):5511-20. doi: 10.4049/jimmunol.1200324.
doi: 10.4049/jimmunol.1200324 pmid: 22547693 |
| 19. |
Gao J, Chen T, Hu G, et al. Nectin-like molecule 1 is a glycoprotein with a single N-glycosylation site at N290KS which influences its adhesion activity. Biochim Biophys Acta,2008; 1778(6):1429-35. doi: 10.1016/j.bbamem.2008.03.013.
doi: 10.1016/j.bbamem.2008.03.013 pmid: 18420026 |
| 20. |
Chang VT, Crispin M, Aricescu AR, et al. Glycoprotein structural genomics: solving the glycosylation problem. Structure,2007; 15(3):267-73. doi: 10.1016/j.str.2007.01.011.
doi: 10.1016/j.str.2007.01.011 pmid: 17355862 |
| 21. |
Elbein AD, Tropea JE, Mitchell M, et al. Kifunensine, a potent inhibitor of the glycoprotein processing mannosidase I. J Biol Chem,1990; 265(26):15599-605.
doi: 10.1016/0014-5793(90)81275-S pmid: 2144287 |
| 22. |
Flanagan JG, Leder P . The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell,1990; 63(1):185-94. doi: 10.1016/0092-8674(90)90299-T.
doi: 10.1016/0092-8674(90)90299-T pmid: 1698555 |
| 23. |
Flanagan JG, Cheng HJ . Alkaline phosphatase fusion proteins for molecular characterization and cloning of receptors and their ligands. Methods Enzymol,2000; 327:198-210. doi: 10.1016/S0076-6879(00)27277-7.
doi: 10.1016/S0076-6879(00)27277-7 |
| 24. |
Flanagan JG, Cheng HJ, Feldheim DA, et al. Alkaline phosphatase fusions of ligands or receptors as in situ probes for staining of cells, tissues, and embryos. Methods Enzymol,2000; 327:19-35. doi: 10.1016/S0076-6879(00)27264-9.
doi: 10.1016/S0076-6879(00)27264-9 |
| 25. |
Zhang Y, Chao T, Li R, et al. MicroRNA-128 inhibits glioma cells proliferation by targeting transcription factor E2F3a. J Mol Med (Berl),2009; 87(1):43-51. doi: 10.1007/s00109-008-0403-6.
doi: 10.1007/s00109-008-0403-6 pmid: 18810376 |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
|

