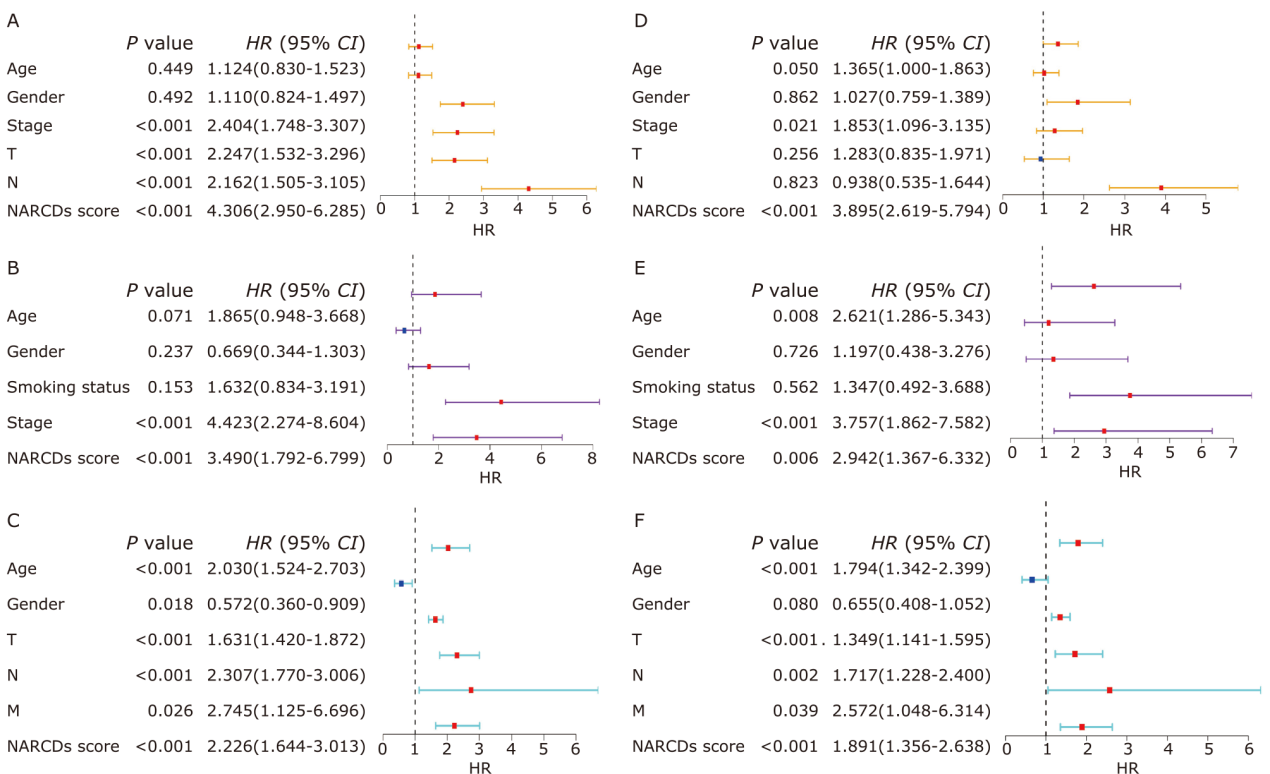
| 1 |
Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018; 553(7689):446-54. doi: 10.1038/nature25183.
|
| 2 |
Miller K D, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019; 69(5):363-85. doi: 10.3322/caac.21565.
|
| 3 |
Gao W, Wang X, Zhou Y, et al. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther 2022; 7(1):196. doi: 10.1038/s41392-022-01046-3.
|
| 4 |
Liu J, Sun M, Sun Y, et al. TMEM189 promotes breast cancer through inhibition of autophagy-regulated ferroptosis. Biochem Biophys Res Commun 2022; 622:37-44. doi: 10.1016/j.bbrc.2022.06.024.
|
| 5 |
Xie Y, Zhao Y, Shi L, et al. Gut epithelial TSC1/mTOR controls RIPK3-dependent necroptosis in intestinal inflammation and cancer. J Clin Invest 2020; 130(4):2111-28. doi: 10.1172/jci133264.
pmid: 31961824
|
| 6 |
Carleton G, Lum JJ. Autophagy metabolically suppresses CD8(+) T cell antitumor immunity. Autophagy 2019; 15(9):1648-9. doi: 10.1080/15548627.2019.1628545.
pmid: 31170865
|
| 7 |
Snyder AG, Hubbard NW, Messmer MN, et al. Intratumoral activation of the necroptotic pathway components RIPK1 and RIPK 3 potentiates antitumor immunity. Sci Immunol 2019; 2019; 4(36):eaaw2004. doi: 10.1126/sciimmunol.aaw2004.
|
| 8 |
Wang Q, Wang Y, Ding J, et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature 2020; 579(7799):421-6. doi: 10.1038/s41586-020-2079-1.
|
| 9 |
Xu C, Sun S, Johnson T, et al. The glutathione peroxidase Gpx 4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep 2021; 35(11):109235. doi: 10.1016/j.celrep.2021.109235.
|
| 10 |
Fang Q, Chen H. Development of a novel autophagy-related prognostic signature and nomogram for hepatocellular carcinoma. Front Oncol 2020; 10:591356. doi: 10.3389/fonc.2020.591356.
|
| 11 |
Xu D, Ji Z, Qiang L. Molecular characteristics, clinical implication, and cancer immunity interactions of pyroptosis-related genes in breast cancer. Front Med (Lausanne) 2021; 8:702638. doi: 10.3389/fmed.2021.702638.
|
| 12 |
Zhang X, Yang Q. A pyroptosis-related gene panel in prognosis prediction and immune microenvironment of human endometrial cancer. Front Cell Dev Biol 2021; 9:705828. doi: 10.3389/fcell.2021.705828.
|
| 13 |
Xing M, Li J. Diagnostic and prognostic values of pyroptosis-related genes for the hepatocellular carcinoma. BMC Bioinformatics 2022; 23(1):177. doi: 10.1186/s12859-022-04726-7.
pmid: 35562678
|
| 14 |
Zhang Z, Hu X, Qiu D, et al. Development and validation of a necroptosis-related prognostic model in head and neck squamous cell carcinoma. J Oncol 2022; 2022:8402568. doi: 10.1155/2022/8402568.
|
| 15 |
Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 2013; 14:7. doi: 10.1186/1471-2105-14-7.
pmid: 23323831
|
| 16 |
Yoshihara K, Shahmoradgoli M, Martínez E, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun 2013; 4:2612. doi: 10.1038/ncomms3612.
pmid: 24113773
|
| 17 |
Jiang P, Gu S, Pan D, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med 2018; 24(10):1550-8. doi: 10.1038/s41591-018-0136-1.
pmid: 30127393
|
| 18 |
Yang W, Soares J, Greninger P, et al. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res 2013; 41:D955-61. doi: 10.1093/nar/gks1111.
|
| 19 |
von Mering C, Huynen M, Jaeggi D, et al. STRING: a database of predicted functional associations between proteins. Nucleic Acids Res 2003; 31(1):258-61. doi: 10.1093/nar/gkg034.
pmid: 12519996
|
| 20 |
Chin CH, Chen SH, Wu HH, et al. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol 2014; 8 Suppl 4(Suppl 4):S11. doi: 10.1186/1752-0509-8-s4-s11.
|
| 21 |
Han H, Yang C, Ma J, et al.N(7)-methylguanosine tRNA modification promotes esophageal squamous cell carcinoma tumorigenesis via the RPTOR/ULK1/autophagy axis. Nat Commun 2022; 13(1):1478. doi: 10.1038/s41467-022-29125-7.
|
| 22 |
Lan H, Liu Y, Liu J, et al. Tumor-associated macrophages promote oxaliplatin resistance via METTL3-mediated m(6)A of TRAF5 and necroptosis in colorectal cancer. Mol Pharm 2021; 18(3):1026-37. doi: 10.1021/acs.molpharmaceut.0c00961.
|
| 23 |
Xu Y, Lv D, Yan C, et al. METTL3 promotes lung adenocarcinoma tumor growth and inhibits ferroptosis by stabilizing SLC7A11 m(6)A modification. Cancer Cell Int 2022; 22(1):11. doi: 10.1186/s12935-021-02433-6.
|
| 24 |
Li N, Wang J, Zhan X. Identification of immune-related gene signatures in lung adenocarcinoma and lung squamous cell carcinoma. Front Immunol 2021; 12:752643. doi: 10.3389/fimmu.2021.752643.
|
| 25 |
Wu X, Zhu J, Liu W, et al. A novel prognostic and predictive signature for lung adenocarcinoma derived from combined hypoxia and infiltrating immune cell-related genes in TCGA patients. Int J Gen Med 2021; 14:10467-81. doi: 10.2147/ijgm.S342107.
pmid: 35002303
|
| 26 |
Niu N, Zeng J, Ke X, et al. ATIC facilitates cell growth and migration by upregulating Myc expression in lung adenocarcinoma. Oncol Lett 2022; 23(4):131. doi: 10.3892/ol.2022.13251.
pmid: 35251351
|
| 27 |
Li Z, Zhang Y, Zhou Y, et al. Tanshinone IIA suppresses the progression of lung adenocarcinoma through regulating CCNA2-CDK2 complex and AURKA/PLK1 pathway. Sci Rep 2021; 11(1):23681. doi: 10.1038/s41598-021-03166-2.
pmid: 34880385
|
| 28 |
Zhang X, Liu X, Cui W, et al. Sohlh2 alleviates malignancy of EOC cells under hypoxia via inhibiting the HIF1α/CA9 signaling pathway. Biol Chem 2020; 401(2):263-71. doi: 10.1515/hsz-2019-0119.
|
| 29 |
Gan L, Meng J, Xu M, et al. Extracellular matrix protein 1 promotes cell metastasis and glucose metabolism by inducing integrin β4/FAK/SOX2/HIF-1α signaling pathway in gastric cancer. Oncogene 2018; 37(6):744-55. doi: 10.1038/onc.2017.363.
pmid: 29059156
|
| 30 |
Fan CC, Cheng WC, Huang YC, et al. EFHD2 promotes epithelial-to-mesenchymal transition and correlates with postsurgical recurrence of stage I lung adenocarcinoma. Sci Rep 2017; 7(1):14617. doi: 10.1038/s41598-017-15186-y.
|
| 31 |
Jiang X, Li Y, Zhang N, et al. RRM2 silencing suppresses malignant phenotype and enhances radiosensitivity via activating cGAS/STING signaling pathway in lung adenocarcinoma. Cell Biosci 2021; 11(1):74. doi: 10.1186/s13578-021-00586-5.
|
| 32 |
Peng J, Li W, Tan N, et al. USP47 stabilizes BACH1 to promote the Warburg effect and non-small cell lung cancer development via stimulating Hk2 and Gapdh transcription. Am J Cancer Res 2022; 12(1):91-107.
|
| 33 |
Lv X, Yu H, Zhang Q, et al. SRXN1 stimulates hepatocellular carcinoma tumorigenesis and metastasis through modulating ROS/p65/BTG2 signalling. J Cell Mol Med 2020; 24(18):10714-29. doi: 10.1111/jcmm.15693.
|
| 34 |
Yu X, Liu W, Chen S, et al. Immunologically programming the tumor microenvironment induces the pattern recognition receptor NLRC4-dependent antitumor immunity. J Immunother Cancer 2021; 9(1):e001595. doi: 10.1136/jitc-2020-001595.
|
| 35 |
Suzuki S, Venkatesh D, Kanda H, et al. GLS2 is a tumor suppressor and a regulator of ferroptosis in hepatocellular carcinoma. Cancer Res 2022; 82(18):3209-22. doi: 10.1158/0008-5472.Can-21-3914.
|
| 36 |
Lu T, Zheng C, Fan Z. Cardamonin suppressed the migration, invasion, epithelial mesenchymal transition (EMT) and lung metastasis of colorectal cancer cells by down-regulating ADRB2 expression. Pharm Biol 2022; 60(1):1011-21. doi: 10.1080/13880209.2022.2069823.
pmid: 35645356
|
| 37 |
Tang W, Jia P, Zuo L, et al. Suppression of CX3CL 1 by miR-497-5p inhibits cell growth and invasion through inactivating the ERK/AKT pathway in NSCLC cells. Cell Cycle 2022; 21(16):1697-709. doi: 10.1080/15384101.2022.2067438.
|
| 38 |
Duan L, Pang HL, Chen WJ, et al. The role of GDF 15 in bone metastasis of lung adenocarcinoma cells. Oncol Rep 2019; 41(4):2379-88. doi: 10.3892/or.2019.7024.
|
| 39 |
Zhao H, Xu Y, Xie Y, et al. m6A regulators are differently expressed and correlated with immune response of esophageal cancer. Front Cell Dev Biol 2021; 9:650023. doi: 10.3389/fcell.2021.650023.
|
| 40 |
Demelash A, Rudrabhatla P, Pant HC, et al. Achaete-scute homologue-1 (ASH1) stimulates migration of lung cancer cells through Cdk5/p35 pathway. Mol Biol Cell 2012; 23(15):2856-66. doi: 10.1091/mbc.E10-12-1010.
pmid: 22696682
|
| 41 |
Du F, Sun L, Chu Y, et al. DDIT4 promotes gastric cancer proliferation and tumorigenesis through the p53 and MAPK pathways. Cancer Commun (Lond) 2018; 38(1):45. doi: 10.1186/s40880-018-0315-y.
|
| 42 |
Nakae S, Suto H, Iikura M, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol 2006; 176(4):2238-48. doi: 10.4049/jimmunol.176.4.2238.
pmid: 16455980
|
| 43 |
Lorente E, García R, López D. Allele-dependent processing pathways generate the endogenous human leukocyte antigen (HLA) class I peptide repertoire in transporters associated with antigen processing (TAP)-deficient cells. J Biol Chem 2011; 286(44):38054-9. doi: 10.1074/jbc.M111.281808.
pmid: 21914809
|
| 44 |
Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev 2002; 13(2):95-109. doi: 10.1016/s1359-6101(01)00038-7.
|
| 45 |
Vatner RE, Janssen EM. STING, DCs and the link between innate and adaptive tumor immunity. Mol Immunol 2019; 110:13-23. doi: 10.1016/j.molimm.2017.12.001.
pmid: 29273394
|
| 46 |
Kirtonia A, Sethi G, Garg M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell Mol Life Sci 2020; 77(22):4459-83. doi: 10.1007/s00018-020-03536-5.
|
| 47 |
Stenzinger A, Allen JD, Maas J, et al. Tumor mutational burden standardization initiatives: recommendations for consistent tumor mutational burden assessment in clinical samples to guide immunotherapy treatment decisions. Genes Chromosomes Cancer 2019; 58(8):578-88. doi: 10.1002/gcc.22733.
|
| 48 |
Xu J, Zhang Y, Jia R, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res 2019; 25(2):515-23. doi: 10.1158/1078-0432.Ccr-18-2484.
pmid: 30348638
|
| 49 |
Ning XH, Li NY, Qi YY, et al. Identification of a hypoxia-related gene model for predicting the prognosis and formulating the treatment strategies in kidney renal clear cell carcinoma. Front Oncol 2021; 11:806264. doi: 10.3389/fonc.2021.806264.
|
| 50 |
Zhou G, Liu Z, Myers JN. TP53 mutations in head and neck squamous cell carcinoma and their impact on disease progression and treatment response. J Cell Biochem 2016; 117(12):2682-92. doi: 10.1002/jcb.25592.
pmid: 27166782
|
| 51 |
Canale M, Andrikou K, Priano I, et al. The role of TP 53 mutations in EGFR-mutated non-small-cell lung cancer: clinical significance and implications for therapy. Cancers (Basel) 2022; 14(5):1143. doi: 10.3390/cancers14051143.
|
 ),王文锐1,4,*(
),王文锐1,4,*( )
)
 ),Wen-Rui Wang1,4,*(
),Wen-Rui Wang1,4,*( )
)