| 1. |
Vehik K and Dabelea D. The changing epidemiology of type 1 diabetes: why is it going through the roof? Diabetes Metab Res Rev 2011; 27:3-13.
|
| 2. |
Vallon V.The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med 2015; 66:255-70.
|
| 3. |
Saulsberry WJ, Coleman CI, Mearns ES, et al.Comparative efficacy and safety of antidiabetic drug regimens added to stable and inadequate metformin and thiazolidinedione therapy in type 2 diabetes. Int J Clin Pract 2015; 69:1221-35.
|
| 4. |
Diabetes mellitus. Report of a WHO Study Group. World Health Organ Tech Rep Ser 1985; 727:1-113.
|
| 5. |
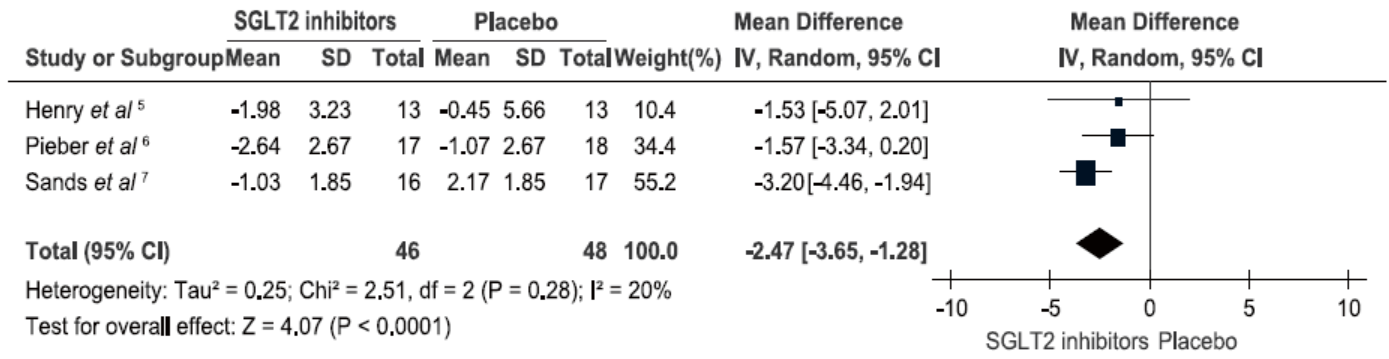
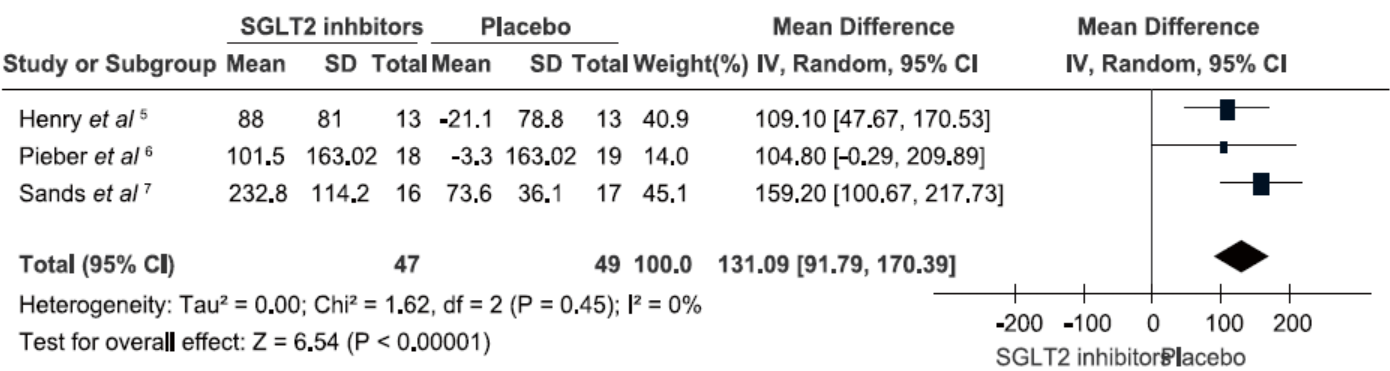
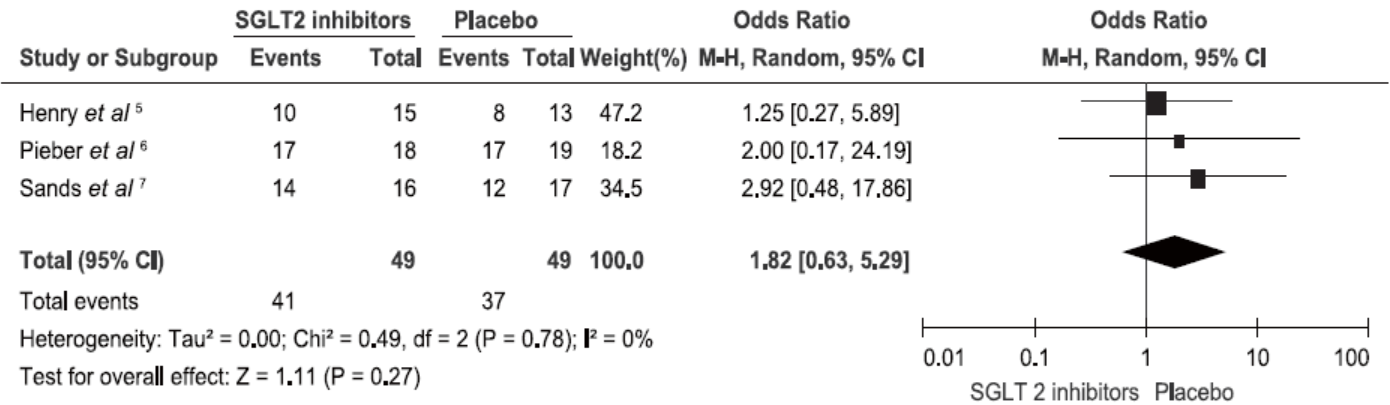
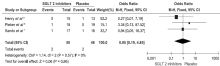
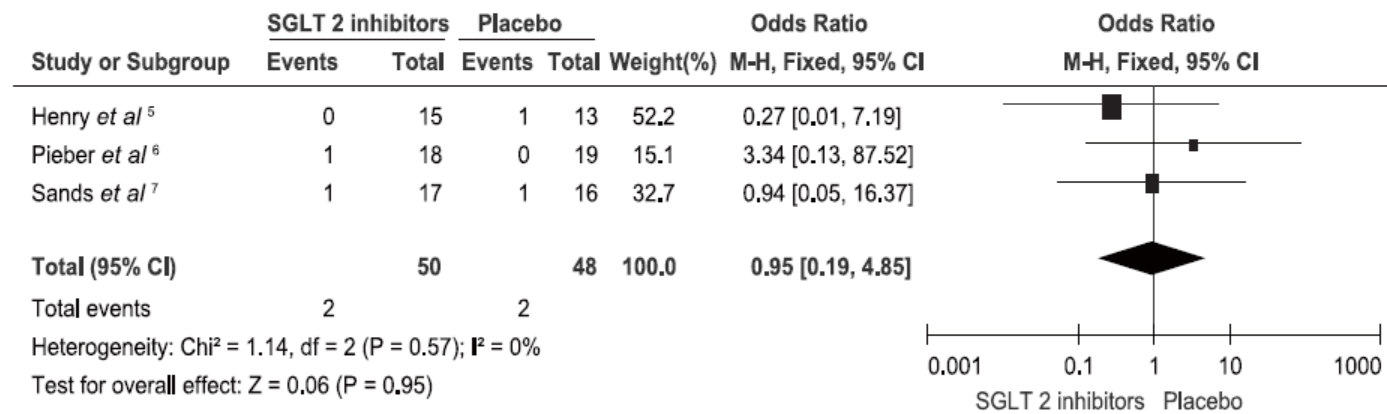
Henry RR, Rosenstock J, Edelman S, et al.Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care 2015; 38:412-9.
|
| 6. |
Pieber TR, Famulla S, Eilbracht J, et al.Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4-week, randomized, placebo-controlled trial (EASE-1). Diabetes Obes Metab 2015; 17:928-35.
|
| 7. |
Sands AT, Zambrowicz BP, Rosenstock J, et al.Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct therapy to insulin in type 1 diabetes. Diabetes Care 2015; 38:1181-8.
|
| 8. |
Bell DS.Case reports that illustrate the efficacy of SGLT2 inhibitors in the type 1 diabetic patient. Case Rep Endocrinol 2015; 2015:676191.
|
| 9. |
Vouyiouklis M.Canagliflozin: improving diabetes by making urine sweet. Cleve Clin J Med 2013; 80:683-7.
|
| 10. |
Kirkham JJ, Dwan KM, Altman DG, et al.The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010; 340:c365.
|
| 11. |
Tanaka M.Relationship between fasting and 2-hour postprandial plasma glucose levels and vascular complications in patients with type 2 diabetes mellitus. J Int Med Res 2012; 40:1295-303.
|
| 12. |
Tahrani AA, Barnett AH, Bailey CJ.SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol 2013; 1:140-51.
|
| 13. |
Palmer SC, Mavridis D, Nicolucci A, et al.Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis. JAMA 2016; 316:313-24.
|
| 14. |
Comee M, Peters A.The changing therapeutic armamentarium for patients with type 1 diabetes. Curr Opin Endocrinol Diabetes Obes 2016; 23:106-10.
|
| 15. |
Scheen AJ.SGLT2 inhibition: efficacy and safety in type 2 diabetes treatment. Expert Opin Drug Saf 2015; 14: 1879-904.
|
| 16. |
Hinnen D.Glucuretic effects and renal safety of dapagliflozin in patients with type 2 diabetes. Ther Adv Endocrinol Metab 2015; 6:92-102.
|
| 17. |
Handelsman Y, Henry RR, Bloomgarden ZT, et al.American Association of Clinical Endocrinologists and American College of Endocrinology Position Statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr Pract 2016; 22:753-62.
|
 ), Wang Bo2, Chen Shi1, Zhu Huijuan1
), Wang Bo2, Chen Shi1, Zhu Huijuan1