Chinese Medical Sciences Journal ›› 2023, Vol. 38 ›› Issue (2): 77-93.doi: 10.24920/004213
• Guideline & Consensus • Next Articles
Chinese Guideline on the Management of Polypoidal Choroidal Vasculopathy (2022)
You-Xin Chen1, 2, Yu-Qing Zhang3, 4, 5, 6, Chang-Zheng Chen7, Hong Dai8, Su-Yan Li9, Xiang Ma10, Xiao-Dong Sun11, Shi-Bo Tang12, Yu-Sheng Wang13, Wen-Bin Wei14, Feng Wen15, Ge-Zhi Xu16, Wei-Hong Yu1, 2, Mei-Xia Zhang17, Ming-Wei Zhao18, Yang Zhang19, Fang Qi19, Xun Xu20, *( ), Xiao-Xin Li21, 22, *(
), Xiao-Xin Li21, 22, *( )
)
- 1Department of Ophthalmology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
2Key Laboratory of Ocular Fundus Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
3Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON, Canada
4Ningbo Nottingham GRADE center, University of Nottingham, Ningbo, China
5Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Beijing, China
6CEBIM (Center for Evidence Based Integrative Medicine)-Clarity Collaboration, Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
7Department of Ophthalmology, Renmin Hospital of Wuhan University, Wuhan, China
8Department of Ophthalmology, Beijing Hospital, Beijing, China
9Department of Ophthalmology, Xuzhou Municipal Hospital Affiliated to Xuzhou medical University, Xuzhou, China
10Department of Ophthalmology, the First Affiliated Hospital of Dalian Medical University, Dalian, China
11Department of Ophthalmology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
12Aire Eye Hospital, Changsha, China
13Department of Ophthalmology, Xijing Hospital, Fouth Military Medical University, Xi‘an, China
14Department of Ophthalmology, Beijing Tongren Hospital, Beijing, China
15State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, China
16Ophthalmology, the Affiliated Eye and ENT Hospital, Shanghai Medical School, Fudan University, Shanghai, China
17Department of Ophthalmology, West China School of Medicine, West China Hospital, Sichuan University, Chengdu, China
18Department of Ophthalmology, Peking University People’s Hospital, Beijing, China
19Department of Ophthalmology, Peking University People’s Hospital, Beijing, China
20Department of Ophthalmology, Shanghai General Hospital, Shanghai, China
21Eye Center of Xiamen University, Xiamen, China
22People Eye Center of People’s Hospital, Peking University, Beijing, China
-
Accepted:2023-04-06Published:2023-06-30Online:2023-05-31 -
Contact:*Xun Xu, E-mail:drxuxun@sjtu.edu.cn ; Xiaoxin Li, E-mail:dr_lixiaoxin@163.com
Cite this article
You-Xin Chen, Yu-Qing Zhang, Chang-Zheng Chen, Hong Dai, Su-Yan Li, Xiang Ma, Xiao-Dong Sun, Shi-Bo Tang, Yu-Sheng Wang, Wen-Bin Wei, Feng Wen, Ge-Zhi Xu, Wei-Hong Yu, Mei-Xia Zhang, Ming-Wei Zhao, Yang Zhang, Fang Qi, Xun Xu, Xiao-Xin Li. Chinese Guideline on the Management of Polypoidal Choroidal Vasculopathy (2022)[J].Chinese Medical Sciences Journal, 2023, 38(2): 77-93.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
"
| BOX 1: SUMMARY OF RECOMMENDATIONS | |
|---|---|
| 1 | For treatment-na?ve polypoidal choroidal vasculopathy (PCV) patients with inactive polypoidal lesions, the guideline panel suggests observation over the initiation of treatment (conditional recommendation, very low certainty in the estimated effects). |
| Remarks: Close follow-up and monitoring are essential, especially for patients with high-risk factors (such as cigarette smoking, higher body mass index, and abnormal serum levels of inflammatory markers). | |
| 2 | For treatment-na?ve PCV patients, the guideline panel suggests either anti-VEGF monotherapy or combined anti-VEGF and PDT rather than PDT monotherapy (conditional recommendation, low to very low certainty in the estimated effects). |
| Remarks: The choice may depend on the patient’s condition (such as the size or location of polypoidal lesions and the height of PED) or specific types of anti-VEGF agents. | |
| 3 | For PCV patients who plan to initiate anti-VEGF combined with PDT treatment, the guideline panel suggests later/rescue PDT over initial PDT (conditional recommendation, low certainty in the estimated effects). |
| Remarks: The timing of later PDT may be at least after three months of anti-VEGF according to treatment criteria of PDT (such as if polypoidal lesions are seen with subretinal fluid on the ICGA images obtained) | |
| 4 | For PCV patients who plan to initiate the treatment with anti-VEGF, the guideline panel suggests treat and extend (T&E) over the pro re nata (PRN) regimen following three monthly loading doses (conditional recommendation, very low certainty in the estimated effects). |
| Remarks: The T&E regimen increases the number of injections compared to the PRN regimen, although it reduces the number of visits. The follow-up should consider the morphological changes of the fundus and pay more attention to the functional or conscious symptoms of the affected eye. The interval of T&E can be referred to in the ALTAIR study. | |
| 5 | For PCV patients with persistent subretinal fluid (SRF) or intraretinal fluid (IRF) on optical coherence tomography (OCT) after three monthly anti-VEGF treatments, the guideline panel suggests proceeding with anti-VEGF treatment over observation (conditional recommendation, very low certainty of the estimated effects). |
| Remarks: Clinicians should closely monitor the change in fundus morphology and function of the affected eye (or subjective symptoms) during follow-up and may consider stopping treatment when no clear benefit to visual acuity with further injection is expected, such as extensive subretinal scar formation. | |
| 6 | For PCV patients with massive subretinal hemorrhage (equal or more than four optic disc areas) involving the central macula within the onset of 14 days, the panel suggests vitrectomy in combination with tissue-plasminogen activator (tPA) intraocular injection and gas tamponade over anti-VEGF monotherapy (conditional recommendation, very low certainty in the estimated effects). |
| Remarks: Surgery may also benefit PCV patients with subretinal hemorrhage combined with vitreous hemorrhage; clinicians might consider using complementary therapy (e.g., pneumatic displacement, anti-VEGF, PDT, and tPA). | |

Figure 1.
Logic model for identification of important clinical questions on clinical management of patients wth PCV. BVN: branching vascular network; IRF: intraretinal fluid; PCV: polypoidal choroidal vasculopathy; PDT: photodynamic therapy; PRN: pro re nata; SRF: subretinal fluid; T&E: treat and extend; VEGF: vascular endothelial growth factor; OCT: optical coherence tomography."

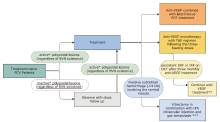
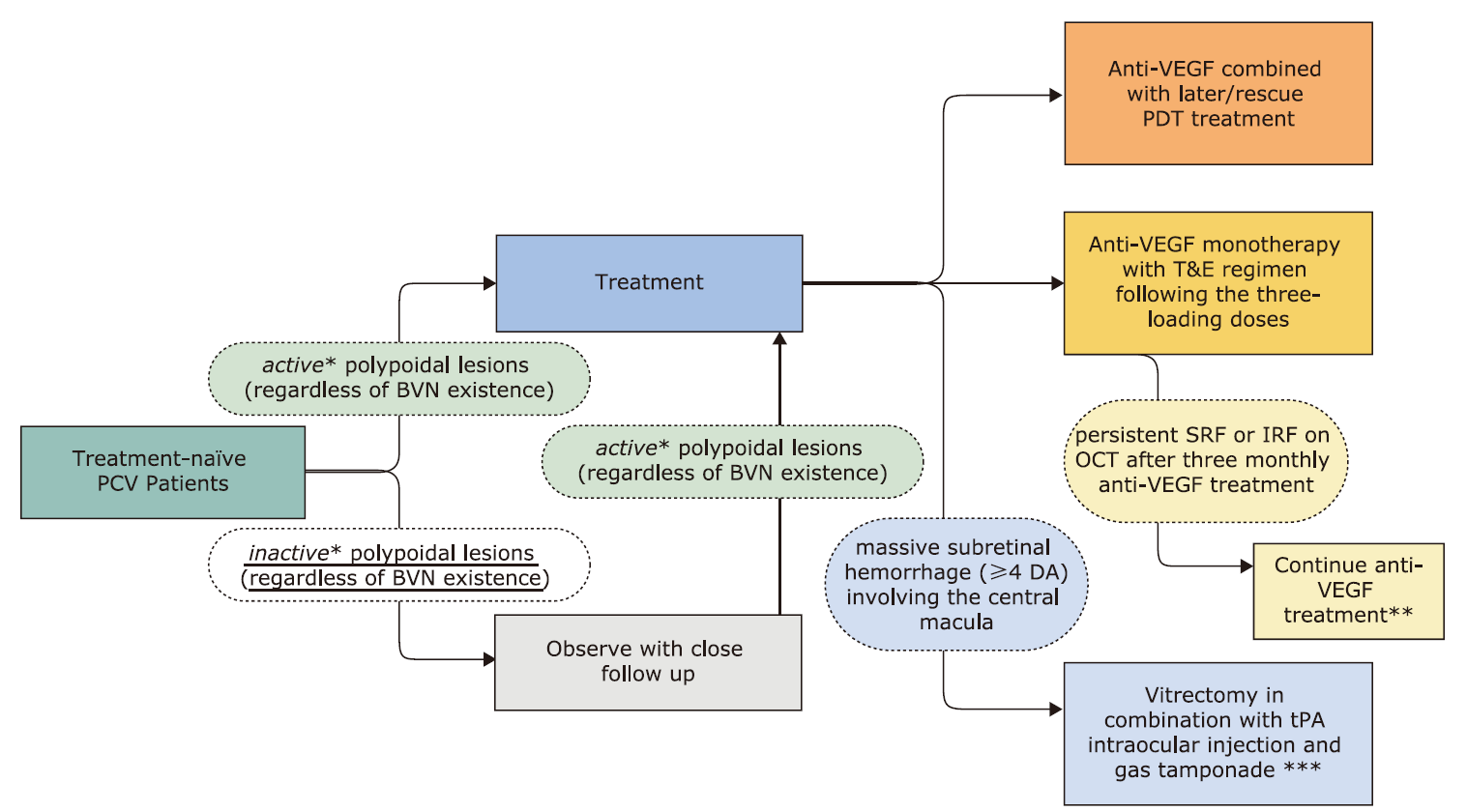
Figure 2.
Recommendations for clinical questions (listed in Figure 1) on clinical management of PCV. BVN: branching vascular network; DA: disc area; PCV: polypoidal choroidal vasculopathy; SRF: subretinal fluid; IRF: intraretinal fluid; tPA: tissue plasminogen activator; PDT: photodynamic therapy; T&E: treat and extend; VEGF: vascular endothelial growth factor. *active: subretinal fluid, intraretinal fluid or subretinal hemorrhage or vitreous hemorrhage on OCT or fundoscopy; inactive: without subretinal fluid, intraretinal fluid or subretinal hemorrhage or vitreous hemorrhage on OCT or fundoscopy **Clinicians should closely observe changes in fundus morphology and function of the affected eye (or subjective symptoms) during follow-up, and may consider to stop treatment in cases when no clear benefit to visual acuity with further injection is expected, such as large subretinal scar formation. ***For patients with PCV and subretinal hemorrhage combined with vitreous hemorrhage, the recommendations apply as well."
| 1 |
Spaide RF, Jaffe GJ, Sarraf D, et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020 ;127(5):616-36. doi: 10.1016/j.ophtha.2019.11.004.
doi: 10.1016/j.ophtha.2019.11.004 |
| 2 |
Spaide RF, Yannuzzi LA, Slakter JS, et al. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 1995 ; 15(2):100-10. doi: 10.1097/00006982-199515020-00003.
doi: 10.1097/00006982-199515020-00003 |
| 3 |
Han LH, Yuan LF, Liang X, et al. Combined therapy versus anti-vascular endothelial growth factor monotherapy for polypoidal choroidal vasculopathy: a meta-analysis. Int J Ophthalmol 2017 ; 10(8):1280-9. doi: 10.18240/ijo.2017.08.16.
doi: 10.18240/ijo.2017.08.16 |
| 4 |
Qu J, Cheng Y, Li X, et al. Efficacy of intravitreal injection of conbercept in polypoidal choroidal vasculopathy: Subgroup analysis of the aurora study. Retina 2016 ; 36(5):926-37. doi: 10.1097/iae.0000000000000875.
doi: 10.1097/iae.0000000000000875 |
| 5 |
Li XX, Zhu Q, Egger A, et al. Two different treatment regimens of ranibizumab 0.5 mg for neovascular age-related macular degeneration with or without polypoidal choroidal vasculopathy in Chinese patients: results from the phase IV, randomized, DRAGON study. Acta Ophthalmol 2021 ; 99(3): e336-e45. doi: 10.1111/aos.14588.
doi: 10.1111/aos.14588 |
| 6 |
Cheung CMG, Yanagi Y, Akiba M, et al. Improved detection and diagnosis of polypoidal choroidal vasculopathy using a combination of optical coherence tomography and optical coherence tomography angiography. Retina 2019 ; 39(9):1655-63. doi: 10.1097/iae.0000000000002228.
doi: 10.1097/iae.0000000000002228 |
| 7 |
Koh AHC, Chen LJ, Chen SJ, et al. Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina 2013 ; 33(4):686-716. doi: 10.1097/IAE.0b013e3182852446.
doi: 10.1097/IAE.0b013e3182852446 |
| 8 |
Bo Q, Yan Q, Shen M, et al. Appearance of polypoidal lesions in patients with polypoidal choroidal vasculopathy using swept-source optical coherence tomographic angiography. JAMA Ophthalmol 2019 ; 137(6):642-50. doi: 10.1001/jamaophthalmol.2019.0449.
doi: 10.1001/jamaophthalmol.2019.0449 |
| 9 |
De Carlo TE, Kokame GT, Kaneko KN, et al. Sensitivity and specificity of detecting polypoidal choroidal vasculopathy with en face optical coherence tomography and optical coherence tomography angiography. Retina 2019 ; 39(7):1343-52. doi: 10.1097/iae.0000000000002139.
doi: 10.1097/iae.0000000000002139 |
| 10 |
Chen LJ, Cheng CK, Yeung L, et al. Management of polypoidal choroidal vasculopathy: Experts consensus in Taiwan. J Formos Med Assoc 2019 ; 19(2):569-76. doi: 10.1016/j.jfma.2019.04.012.
doi: 10.1016/j.jfma.2019.04.012 |
| 11 |
Cheung CMG, Lai TYY, Ruamviboonsuk P, et al. Polypoidal choroidal vasculopathy: definition, pathogenesis, diagnosis, and management. Ophthalmology 2018 ; 125(5):708-24. doi: 10.1016/j.ophtha.2017.11.019.
doi: 10.1016/j.ophtha.2017.11.019 |
| 12 |
Institute of Medicine US committee on Standards for developing Trustworthy clinical practice Guideline. Graham R, Greenfield S, Steinberg E. Clinical Practice Guidelines We Can Trust. Washington(DC): National Academies Press; 2011. doi: 10.17226/13058.
doi: 10.17226/13058 |
| 13 |
Alonso-Coello P, Oxman AD, Moberg J, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ 2016; 353: i2089. doi: 10.1136/bmj.i2089.
doi: 10.1136/bmj.i2089 |
| 14 |
Guyatt GH, Oxman AD, Kunz R, et al. GRADE Working Group. GRADE guidelines: 7. Rating the quality of evidence-inconsistency. J Clin Epidemiol 2011; 64:1294-302. doi: 10.1016/j.jclinepi.2011.03.017.
doi: 10.1016/j.jclinepi.2011.03.017 |
| 15 |
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924-26. doi: 10.1136/bmj.39489.470347.AD.
doi: 10.1136/bmj.39489.470347.AD |
| 16 |
Schunemann HJ, Mustafa R, Brozek J, et al. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. J Clin Epidemiol 2016; 76:89-98. doi: 10.1016/j.jclinepi.2016.01.032.
doi: 10.1016/j.jclinepi.2016.01.032 pmid: 26931285 |
| 17 | Higgins JPT, Deeks JJ, Altman DG. Editors. Special topics in statistics. In: Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0. updated March 2011. The cochrane collaberation 2011. Avail-able from www.handbook.cochrane.org. Accessed: October 17, 2019. |
| 18 |
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25:603-5. doi: 10.1007/s10654-010-9491-z.
doi: 10.1007/s10654-010-9491-z pmid: 20652370 |
| 19 | Schunemann HJ, Oxman AD, Vist GE, et al. 12 Interpreting results and drawing conclusions. In: Cochrane handbook for systematic reviews of interventions (lst ed). New York: Wiley; 2008.p359. |
| 20 |
Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64:401-6. doi: 10.1016/j.jclinepi.2010.07.015.
doi: 10.1016/j.jclinepi.2010.07.015 pmid: 21208779 |
| 21 |
Cheung CM, Laude A, Yeo I, et al. Systemic, ocular and genetic risk factors for age-related macular degeneration and polypoidal choroidal vasculopathy in Singaporeans. Sci Rep 2017; 7:41386. doi: 10.1038/srep41386.
doi: 10.1038/srep41386 pmid: 28120909 |
| 22 |
Fujiwara K, Yasuda M, Hata J, et al. Prevalence and risk factors for polypoidal choroidal vasculopathy in a general Japanese population: the hisayama study. Semin Ophthalmol 2018 ; 33(6):813-19. doi: 10.1080/08820538.2018.1506483.
doi: 10.1080/08820538.2018.1506483 |
| 23 |
Meng Q, Huang L, Sun Y, et al. Effect of high-density lipoprotein metabolic pathway gene variations and risk factors on neovascular age-related macular degeneration and polypoidal choroidal vasculopathy in China. PLoS One 2015 ; 10(12):e0143924. doi: 10.1371/journal.pone.0143924.
doi: 10.1371/journal.pone.0143924 |
| 24 |
Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Archiv Ophthalmol 2003 ; 121(10):1392-96. doi: 10.1001/archopht.121.10.1392.
doi: 10.1001/archopht.121.10.1392 |
| 25 |
Woo SJ, Ahn J, Morrison MA, et al. Analysis of genetic and environmental risk factors and their interactions in korean patients with age-related macular degeneration. PLoS One 2015 ; 10(7): e0132771. doi: 10.1371/journal.pone.0132771.
doi: 10.1371/journal.pone.0132771 |
| 26 |
Kikuchi M, Nakamura M, Ishikawa K, et al. Elevated C-reactive protein levels in patients with polypoidal choroidal vasculopathy and patients with neovascular age-related macular degeneration. Ophthalmology 2007 ; 114(9):1722-27. doi: 10.1016/j.ophtha.2006.12.021.
doi: 10.1016/j.ophtha.2006.12.021 |
| 27 |
Sakurada Y, Nakamura Y, Yoneyama S, et al. Aqueous humor cytokine levels in patients with polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Ophthalmic Res 2015 ; 53(1):2-7. doi: 10.1159/000365487.
doi: 10.1159/000365487 |
| 28 |
Okubo A, Arimura N, Abematsu N, et al. Predictable signs of benign course of polypoidal choroidal vasculopathy: based upon the long-term observation of non-treated eyes. Acta Ophthalmol 2010; 88:e107-e14. doi: 10.1111/j.1755-3768.2009.01850.x.
doi: 10.1111/j.1755-3768.2009.01850.x |
| 29 |
Zeng R, Zhang X, Li M, et al. Pilot study of inactive polypoidal lesions in polypoidal choroidal vasculopathy. Eur J Ophthalmol 2015 ; 25(3):222-28. doi: 10.5301/ejo.5000532.
doi: 10.5301/ejo.5000532 |
| 30 |
Koh A, Lee W, Chen L, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 2012 ; 32(8):1453-64. doi: 10.1097/IAE.0b013e31824f91e8.
doi: 10.1097/IAE.0b013e31824f91e8 |
| 31 |
Oishi A, Kojima H, Mandai M, et al. Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12-month LAPTOP study results. Am J Ophthalmol 2013 ; 156(4):644-51. doi: 10.1016/j.ajo.2013.05.024.
doi: 10.1016/j.ajo.2013.05.024 |
| 32 | Guo Y. [Photodynamic therapy combined with intravitreal injection of ranibizumab for polypoidal choroidal vasculopathy]. [dissertation] Nanhua University; 2014. |
| 33 |
Zhao J, Ma LB. [Efficacy of intravitreal ranibizumab in combination with photodynamic therapy on polypoid choroidal vasculopathy]. Chin J Ophthalmol 2016 ;26(6):387-90. Chinese. doi: 10.13444/j.cnki.zgzyykzz.2016.06.010.
doi: 10.13444/j.cnki.zgzyykzz.2016.06.010 |
| 34 |
Lai KB, Li Y, Zhou LJ, et al. Comparison of the effects of photodynamic therapy, intravitreal ranibizumab and combination for polypoidal choroidal vasculopathy under 1+PRN regimen. BMC Ophthalmol 2018; 18:9. doi: 10.1186/s12886-018-0801-7.
doi: 10.1186/s12886-018-0801-7 |
| 35 |
Lee WK, Iida T, Ogura Y, et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study: a randomized clinical trial. JAMA Ophthalmol 2018 ; 136(7):786-93. doi: 10.1001/jamaophthalmol.2018.1804.
doi: 10.1001/jamaophthalmol.2018.1804 |
| 36 |
Wong T, Ogura Y, Lee W, et al. Efficacy and Safety of intravitreal aflibercept for polypoidal choroidal vasculopathy: two-year results of the aflibercept in polypoidal choroidal vasculopathy study. Am J Ophthalmol 2019; 204:80-9. doi: 10.1016/j.ajo.2019.02.027.
doi: S0002-9394(19)30086-8 pmid: 30849345 |
| 37 |
Koh A, Lai T, Takahashi K, et al. Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial. JAMA Ophthalmol 2017 ; 135(11):1206-13. doi: 10.1001/jamaophthalmol.2017.4030.
doi: 10.1001/jamaophthalmol.2017.4030 |
| 38 |
Yoon LJ, Young LS, June-Gone K, et al. Intravitreal bevacizumab alone versus in combination with photodynamic therapy for the treatment of neovascular maculopathy in patients aged 50 years or older: 1-year results of a prospective clinical study. Acta Ophthalmol 2012; 90: 61-7. doi: 10.1111/j.1755-3768.2009.01841.x.
doi: 10.1111/j.1755-3768.2009.01841.x pmid: 20337606 |
| 39 |
Li J, Sun JH, Li B, et al. Intravitreal ranibizumab injection combined with photodynamic therapy for polypoidal choroidal vasculopathy. Exp Ther Med 2018 ; 15(2):1546-51. doi: 10.3892/etm.2017.5565.
doi: 10.3892/etm.2017.5565 |
| 40 |
Guo H, Zhao M, Yan B. [Comparison of visual prognosis between vitreous cavity injection of ranibizumab and vitreous cavity injection of ranibizumab combined with photodynamic therapy for polypoid choroidal vasculopathy]. J Clin Med Literat 2016 ; 3(58):11637. Chinese. doi: 10.3877/j.issn.2095-8242.2016.58.142.
doi: 10.3877/j.issn.2095-8242.2016.58.142 |
| 41 |
Huang Z, Ding Q, Yan M, et al. Short-term efficacy of conbercept and ranibizumab for polypoidal choroidal vasculopathy. Retina 2019 ; 39(5):889-95. doi: 10.1097/iae.0000000000002035.
doi: 10.1097/iae.0000000000002035 |
| 42 |
Kitahashi M, Baba T, Sakurai M, et al. Pneumatic displacement with intravitreal bevacizumab for massive submacular hemorrhage due to polypoidal choroidal vasculopathy. Clin Ophthalmol 2014; 8:485-92. doi: 10.2147/opth.s55413.
doi: 10.2147/opth.s55413 |
| 43 |
Gomi F, Oshima Y, Mori R, et al. Initial versus delayed photodynamic therapy in combination with ranibizumab for treatment of polypoidal choroidal vasculopathy. Retina 2015 ; 35(8):1569-76. doi: 10.1097/IAE.0000000000000526.
doi: 10.1097/IAE.0000000000000526 |
| 44 |
Ohji M, Takahashi K, Okada AA, et al. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR : a randomized controlled trial. Adv Ther 2020; 37(3):1173-87. doi: 10.1007/s12325-020-01236-x.
doi: 10.1007/s12325-020-01236-x pmid: 32016788 |
| 45 |
Eldem BM, Muftuoglu G, Topbas S, et al. A randomized trial to compare the safety and efficacy of two ranibizumab dosing regimens in a Turkish cohort of patients with choroidal neovascularization secondary to AMD. Acta ophthalmol 2015 ; 93(6):e458-64. doi: 10.1111/aos.12540.
doi: 10.1111/aos.12540 |
| 46 |
Aurell S, Sjovall K, Paul A, et al. Better visual outcome at 1 year with antivascular endothelial growth factor treatment according to treat-and-extend compared with pro re nata in eyes with neovascular age-related macular degeneration. Acta Ophthalmol 2019 ; 97(5):519-24. doi: 10.1111/aos.13989.
doi: 10.1111/aos.13989 |
| 47 |
Garweg Justus G, Niderprim Sophie A, Russ Hanna M, et al. Comparison of strategies of treatment with ranibizumab in newly-diagnosed cases of neovascular age-related macular degeneration. J Ocular Pharmac Therapeut 2017 ; 33(10):773-8. doi: 10.1089/jop.2017.0006.
doi: 10.1089/jop.2017.0006 |
| 48 |
Hanemoto T, Hikichi Y, Kikuchi N, et al. The impact of different anti-vascular endothelial growth factor treatment regimens on reducing burden for caregivers and patients with wet age-related macular degeneration in a single-center real-world Japanese setting. PLoS One 2017 ; 12(12): e0189035. doi:10.1371/journal.pone.0189035.
doi: 10.1371/journal.pone.0189035 |
| 49 |
Oubraham H, Cohen SY, Samimi S, et al. Inject and extend dosing versus dosing as needed: a comparative retrospective study of ranibizumab in exudative age-related macular degeneration. Retina 2011 ; 31(1):26-30. doi: 10.1097/IAE.0b013e3181de5609.
doi: 10.1097/IAE.0b013e3181de5609 |
| 50 |
Johnston RL, Carius HJ, Skelly A, et al. A retrospective study of ranibizumab treatment regimens for neovascular age-related macular degeneration (nAMD) in Australia and the United Kingdom. Adv Ther 2017 ; 34(3):703-12. doi: 10.1007/s12325-017-0483-1.
doi: 10.1007/s12325-017-0483-1 |
| 51 |
Hatz K, Prunte C. Treat and Extend versus Pro Re Nata regimens of ranibizumab in neovascular age-related macular degeneration: a comparative 12 Month study. Acta ophthalmol 2017 ; 95(1):e67-e72. doi: 10.1111/aos.13031.
doi: 10.1111/aos.13031 |
| 52 |
Spooner K, Fraser-Bell S, Cozzi M, et al. Macular atrophy in eyes with neovascular age-related macular degeneration treated with vascular endothelial growth factor inhibitors using a treat-and-extend or pro re nata regimen: 4-year results of the MANEX study. Ophthalmology 2020 ; 127(12):1663-73. doi: 10.1016/j.ophtha.2020.06.019.
doi: 10.1016/j.ophtha.2020.06.019 |
| 53 |
Augsburger M, Sarra GM, Imesch P. Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age-related macular degeneration: a comparative study. Graefes Arch Clin Exp Ophthalmol 2019 ; 257(9):1889-95. doi: 10.1007/s00417-019-04404-0.
doi: 10.1007/s00417-019-04404-0 |
| 54 |
Saito M, Kano M, Itagaki K, et al. Subfoveal choroidal thickness in polypoidal choroidal vasculopathy after switching to intravitreal aflibercept injection. Jpn J Ophthalmol 2015 ; 60(1):35-41. doi: 10.1007/s10384-015-0411-3.
doi: 10.1007/s10384-015-0411-3 |
| 55 |
Moon DRC, Lee DK, Kim SH, et al. Aflibercept treatment for neovascular age-related macular degeneration and polypoidal choroidal vasculopathy refractory to anti-vascular endothelial growth factor. Korean J Ophthalmol 2015 ; 29(4):226-32. doi: 10.3341/kjo.2015.29.4.226.
doi: 10.3341/kjo.2015.29.4.226 |
| 56 |
Azuma K, Obata R, Nomura Y, et al. Angiographic findings of ranibizumab-resistant polypoidal choroidal vasculopathy after switching to a treat-and-extend regimen with intravitreal aflibercept. Retina 2016 ; 36(11):2158-65. doi: 10.1097/IAE.0000000000001047.
doi: 10.1097/IAE.0000000000001047 |
| 57 |
Uyama M, Wada M, Nagai Y, et al. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol 2002 ; 133(5):639-48. doi: 10.1016/s0002-9394(02)01404-6.
doi: 10.1016/s0002-9394(02)01404-6 |
| 58 |
Shin JY, Lee JM, Byeon SH. Anti-vascular endothelial growth factor with or without pneumatic displacement for submacular hemorrhage. Am J Ophthalmol 2015 ;159(5):904-14.e1. doi: 10.1016/j.ajo.2015.01.024.
doi: 10.1016/j.ajo.2015.01.024 |
| 59 | Nam K, Kim JY, Lee SJ, et al. Comparison of visual outcome between intravitreal gas injection with t-PA and intravitreal anti-VEGF injection as an initial treatment for submacular hemorrhage associated with ARMD. Invest Ophthalmol Vis Sci 2019 ;60(9):1176. |
| 60 |
Cho HJ, Koh KM, Kim JH, et al. Intravitreal ranibizumab injections with and without pneumatic displacement for treating submacular hemorrhage secondary to neovascular age-related macular degeneration. Retina 2015 ; 35(2):205-12. doi: 10.1097/iae.0000000000000295.
doi: 10.1097/iae.0000000000000295 |
| 61 |
Kang HG, Kang H, Byeon SH, et al. Long-term visual outcomes for treatment of submacular haemorrhage secondary to polypoidal choroidal vasculopathy. Clin Exp Ophthalmol 2018 ; 46(8):916-25. doi: 10.1111/ceo.13198.
doi: 10.1111/ceo.13198 |
| 62 |
Sniatecki JJ, Ho-Yen G, Clarke B, et al. Treatment of submacular hemorrhage with tissue plasminogen activator and pneumatic displacement in age-related macular degeneration. Eur J Ophthalmol 2019;1120672119891625. doi: 10.1177/1120672119891625.
doi: 10.1177/1120672119891625 |
| 63 |
Cho HJ, Koh KM, Kim HS, et al. Anti-vascular endothelial growth factor monotherapy in the treatment of submacular hemorrhage secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol 2013 ; 156(3):524-31. e1. doi: 10.1016/j.ajo.2013.04.029.
doi: 10.1016/j.ajo.2013.04.029 |
| 64 |
Isizaki E, Morishita S, Sato T, et al. Treatment of massive subretinal hematoma associated with age-related macular degeneration using vitrectomy with intentional giant tear. Int Ophthalmol 2016 ; 36(2):199-206. doi: 10.1007/s10792-015-0102-6.
doi: 10.1007/s10792-015-0102-6 |
| 65 |
Jung JH, Lee JK, Lee JE, et al. Results of vitrectomy for breakthrough vitreous hemorrhage associated with age-related macular degeneration and polypoidal choroidal vasculopathy. Retina 2010 ; 30(6):865-73. doi: 10.1097/IAE.0b013e3181c969e9.
doi: 10.1097/IAE.0b013e3181c969e9 |
| 66 |
Kim JH, Kim CG, Lee DW, et al. Intravitreal aflibercept for submacular hemorrhage secondary to neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 2020 ; 258(1):107-16. doi: 10.1007/s00417-019-04474-0.
doi: 10.1007/s00417-019-04474-0 |
| 67 |
Kim KH, Kim JH, Chang YS, et al. Clinical outcomes of eyes with submacular hemorrhage secondary to age-related macular degeneration treated with anti-vascular endothelial growth factor. Korean J Ophthalmol 2015 ; 29(5):315-24. doi: 10.3341/kjo.2015.29.5.315.
doi: 10.3341/kjo.2015.29.5.315 |
| 68 |
Kimura S, Morizane Y, Hosokawa MM, et al. Outcomes of vitrectomy combined with subretinal tissue plasminogen activator injection for submacular hemorrhage associated with polypoidal choroidal vasculopathy. Jpn J Ophthalmol 2019 ; 63(5):382-8. doi: 10.1007/s10384-019-00679-2.
doi: 10.1007/s10384-019-00679-2 |
| 69 |
Kimura S, Morizane Y, Matoba R, et al. Retinal sensitivity after displacement of submacular hemorrhage due to polypoidal choroidal vasculopathy: effectiveness and safety of subretinal tissue plasminogen activator. Jpn J Ophthalmol 2017; 61:472-8. doi: 10.1007/s10384-017-0530-0.
doi: 10.1007/s10384-017-0530-0 pmid: 28836011 |
| 70 |
Kitagawa Y, Shimada H, Mori R, et al. Intravitreal tissue plasminogen activator, ranibizumab, and gas injection for submacular hemorrhage in polypoidal choroidal vasculopathy. Ophthalmology 2016 ; 123(6):1278-86. doi: 10.1016/j.ophtha.2016.01.035.
doi: 10.1016/j.ophtha.2016.01.035 |
| 71 |
Kung YH, Wu TT, Hong MC, et al. Intravitreal tissue plasminogen activator and pneumatic displacement of submacular hemorrhage. J Ocul Pharmacol Ther 2010 ; 26(5):469-74. doi: 10.1089/jop.2010.0066.
doi: 10.1089/jop.2010.0066 |
| 72 |
Lin HC, Yang CH, Yang CM, et al. Visual outcomes of vitrectomy for polypoidal choroidal vasculopathy-related breakthrough vitreous haemorrhage. Eye 2014 ; 28(7):797-806. doi: 10.1038/eye.2014.124.
doi: 10.1038/eye.2014.124 |
| 73 |
Lin TC, Hwang DK, Lee FL, et al. Visual prognosis of massive submacular hemorrhage in polypoidal choroidal vasculopathy with or without combination treatment. J Chin Med Assoc 2016 ; 79(3):159-65. doi: 10.1016/j.jcma.2015.11.004.
doi: 10.1016/j.jcma.2015.11.004 |
| 74 |
Stifter E, Michels S, Prager F, et al. Intravitreal bevacizumab therapy for neovascular age-related macular degeneration with large submacular hemorrhage. Am J Ophthalmologica 2007 ; 144(6):886-92. doi: 10.1016/j.ajo.2007.07.034.
doi: 10.1016/j.ajo.2007.07.034 |
| 75 |
Yang Y, Li J, Liang J, et al. [Observation of polypoidal choroidal vasculopathy with vitreous hemorrhage treated by microincision 25G pars plana vitrectomy]. Int Eye Sci 2020 ; 20(3):571-5. Chinese. doi: 10.3980/j.issn.1672-5123.2020.3.39.
doi: 10.3980/j.issn.1672-5123.2020.3.39 |
| 76 |
Baek J, Kim JH, Lee MY, et al. Disease activity after development of large subretinal hemorrhage in polypoidal choroidal vasculopathy. Retina 2018 ; 38(10):1993-2000. doi: 10.1097/iae.0000000000001817.
doi: 10.1097/iae.0000000000001817 |
| 77 |
Bi X, Chen S, Wang Y, et al. [Observation of vitrectomy on polypoidal choroidal vasculopathy with vitreous hemorrhage]. Chin J Practic Ophthalmol 2012; (6):653-6. Chinese. doi: 10.3760/cma.j.issn.1006-4443.2012.06.005.
doi: 10.3760/cma.j.issn.1006-4443.2012.06.005 |
| 78 |
Li YS, Gao RY, Chen H. [Effect of vitrectomy combined with intravitreal Conbercept injection for polypoidal choroidal vasculopathy associated with vitreous hemorrhage]. Int Eye Sci 2017 ;17(1):113-7. Chinese. doi: 10.3980/j.issn.1672-5123.2017.1.30.
doi: 10.3980/j.issn.1672-5123.2017.1.30 |
| 79 | Pang L. [Efficacy of vitrectomy combined with postoperative anti-VEGF drugs in the treatment of PCV combined with vitreous hemorrhage][dissertation]. Nanchang: Nanchang University; 2016. |
| 80 |
She JT, Zhang GM, Zhao TY, et al. [Influence of intravitreal ranibizumab in pars plana vitrectomy on polypoidal choroidal vasculopathy with vitreous hemorrhage]. Chin J Practic Ophthalmol 2015 ; 33(8):913-17. Chinese. doi: 10.3760/cma.j.issn.1006-4443.2015.08.019.
doi: 10.3760/cma.j.issn.1006-4443.2015.08.019 |
| 81 |
Shen J, Xy LP, Meng XJ, et al. [Effect of vitrectomy with intravitreal Conbercept injection for polypoidal choroidal vasculopathy associated with vitreous hemorrhage]. Int Eye Sci 2018 ;18(9):1660-4. Chinese. doi: 10.3980/j.issn.1672-5123.2018.9.23.
doi: 10.3980/j.issn.1672-5123.2018.9.23 |
| 82 |
Zhang S, Zhang J, Xu XY, et al. [The efficacy of intravitreal injection of tissue plasminogen activator, ranibizumab and C3F8 in the treatment of early submacular hemorrhage induce to polypoid choroid vasculopathy]. Chin J Ocul Fundus Dis 2018 ; 34(5):448-52. Chinese. doi: 10.3760/cma.j.issn.1005-1015.2018.05.007.
doi: 10.3760/cma.j.issn.1005-1015.2018.05.007 |
| 83 |
Yan J, Wu JH. [Effects of photodynamic therapy with Ranibizumab on visual acuity and postoperative complications in patients with PCV complicated with vitreous hemorrhage]. Int Eye Sci 2018 ; 18(12):2209-12. Chinese. doi: 10.3980/j.issn.1672-5123.2018.12.20.
doi: 10.3980/j.issn.1672-5123.2018.12.20 |
| 84 |
Kimura S, Morizane Y, Hosokawa M, et al. Submacular hemorrhage in polypoidal choroidal vasculopathy treated by vitrectomy and subretinal tissue plasminogen activator. Am J Ophthalmol 2015 ; 159(4):683-9. doi: 10.1016/j.ajo.2014.12.020.
doi: 10.1016/j.ajo.2014.12.020 |
| 85 |
Kim JH, Chang YS, Lee DW, et al. Quantification of retinal changes after resolution of submacular hemorrhage secondary to polypoidal choroidal vasculopathy. Jpn J Ophthalmol 2018 ; 62(1):54-62. doi: 10.1007/s10384-017-0549-2.
doi: 10.1007/s10384-017-0549-2 |
| 86 |
Cheng Y, Shi X, Qu JF, et al. Comparison of the 1-year outcomes of conbercept therapy between two different angiographic subtypes of polypoidal choroidal vasculopathy. Chin Med J (Engl) 2016 ; 129(21):2610-16. doi: 10.4103/0366-6999.192779.
doi: 10.4103/0366-6999.192779 |
| 87 |
Ye LH, Cai Y, Shi X, et al. One-year results of intravitreal conbercept in treatment-naïve subjects with polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 2021 ; 259(6):1455-62. doi: 10.1007/s00417-020-04988-y.
doi: 10.1007/s00417-020-04988-y |
| 88 |
Qi HJ, Jin EZ, Zhao MW. One-year outcomes of intravitreal conbercept combined rescue therapy for polypoidal choroidal vasculopathy in a Chinese population: a real-life clinical data. Int J Ophthalmol 2019 ; 12(1):51-7. doi: 10.18240/ijo.2019.01.08.
doi: 10.18240/ijo.2019.01.08 |
| 89 |
Peng Y, Zhang X, Li M, et al. Short-term efficacy of intravitreal conbercept in treatment-naive patients with polypoidal choroidal vasculopathy. Drug Des Devel Ther 2018; 12:339-45. doi: 10.2147/dddt.S158368.
doi: 10.2147/dddt.S158368 |
| 90 |
Li F, Ma A, Zhao B. Comparison of the efficacy of three loading doses of intravitreal injection of conbercept with injection combined with PDT for the treatment of PCV. Biomed Res Int 2020; 2020:2428348. doi: 10.1155/2020/2428348.
doi: 10.1155/2020/2428348 |
| [1] | Yuntai Yao, Xin Yuan, Lixian He, Yiping Yu, Yu Du, Gang Liu, Lijuan Tian, Zuxuan Ma, Yongbao Zhang, Jie Ma. Patient Blood Management: Single Center Evidence and Practice at Fuwai Hospital [J]. Chinese Medical Sciences Journal, 2022, 37(3): 246-260. |
| [2] | Yang Zhang, Ailing Bian, Anyi Liang, Fei Mo, Gangwei Cheng. A Case of Refractory Childhood Glaucoma Secondary to Weill-Marchesani Syndrome: Management with Combined CO2 Laser-Assisted Sclerectomy Surgery and Trabeculectomy [J]. Chinese Medical Sciences Journal, 2022, 37(2): 159-163. |
| [3] | Xiaokun Chen, Qi Miao, Tienan Zhu, Chaoji Zhang. Replacement Therapy for Hemophilia Patients Undergoing Cardiac Surgery: Report of Three Cases [J]. Chinese Medical Sciences Journal, 2022, 37(1): 79-81. |
| [4] | Yuntai Yao, Lixian He, Liping Li. Anesthesia Management at Fuwai Hospital:Practice, Evidence and Outcomes [J]. Chinese Medical Sciences Journal, 2021, 36(3): 234-251. |
| [5] | Yi Wang, Zhuhua Zhang, Wei Lian. Sudden Sensorineural Hearing Loss after Pituitary Adenoma Resection—A Case Series with Literature Review [J]. Chinese Medical Sciences Journal, 2021, 36(2): 120-126. |
| [6] | Weijia Wang, Le Shen, Labaciren , Hange Li, Yuelun Zhang, Yuguang Huang. Evaluation of Burnout Among Anesthesiologists Working in Tibet, China: Altitude and Attitude [J]. Chinese Medical Sciences Journal, 2021, 36(2): 97-102. |
| [7] | An Pugen, Zhao Jizhi. Advance in Functional Restoration of Injured Nerve with Low Level Laser and its Utilization in the Dental and Maxillofacial Region [J]. Chinese Medical Sciences Journal, 2020, 35(3): 272-277. |
| [8] | Ma Lulu,Huang Yuguang. Phrenic Nerve Injury Is a Differential Diagnosis of Hypoxemia after Video-Assisted Thoracoscopic Thymectomy: 2 Cases Report and Literature Review [J]. Chinese Medical Sciences Journal, 2020, 35(2): 191-194. |
| [9] | Li Ying, Wang Yan, on behalf of the Refractive Surgery Experts Group, Ocular Microcirculation Branch, Chinese Society of Microcirculation . Chinese Expert Consensus on Perioperative Medication in Laser Corneal Refractive Surgeries (2019) [J]. Chinese Medical Sciences Journal, 2020, 35(1): 1-12. |
| [10] | Gao Yuan,Tan Ke,Sun Jian,Jiang Tao,Zou Xiaowen. Application of Mixed Reality Technology in Visualization of Medical Operations [J]. Chinese Medical Sciences Journal, 2019, 34(2): 103-109. |
| [11] | Bai Bing, Tian Yuan, Zhang Yuelun, Ma Manjiao, Yu Xuerong, Huang Yuguang. Prediction of Hidden Blood Loss During Posterior Spinal Surgery [J]. Chinese Medical Sciences Journal, 2019, 34(1): 38-44. |
| [12] | Li Xiao-feng, Liu Jian-zhou, Zhang Chao-ji, Miao Qi. Unicentric Castleman’s Disease with Cardiovascular Involvement [J]. Chinese Medical Sciences Journal, 2017, 32(3): 198-200. |
| [13] | Zhang Xue, Yu Xuerong, Huang* Yuguang. The Correlation of Indices in r-TEG with Intra-operative Blood Loss in Neurosurgical Patients [J]. Chinese Medical Sciences Journal, 2017, 32(2): 69-74. |
| [14] | Wu Jinjing, Jiang Qi, Zhang Zhen, Luo Huiyu. Intraoperative Right Ventricular Myocardial Infarction in a Geriatric Patient with Hip Fracture: a Case Report [J]. Chinese Medical Sciences Journal, 2017, 32(2): 132-134. |
| [15] | Ge Qu, Xu-lei Cui, Hong-ju Liu, Zhi-gang Ji, Yu-guang Huang. Ultrasound-guided Transversus Abdominis Plane Block Improves Postoperative Analgesia and Early Recovery in Patients Undergoing Retroperitoneoscopic Urologic Surgeries: A Randomized Controlled Double-blinded Trial [J]. Chinese Medical Sciences Journal, 2016, 31(3): 137-141. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|

