Chinese Medical Sciences Journal ›› 2021, Vol. 36 ›› Issue (4): 265-278.doi: 10.24920/003883
• Original Articles • Previous Articles Next Articles
BAG3-Related Myofibrillar Myopathy Presenting as Hypercapnia: A Case Report and Literature Review
Yan Xu1, Shixuan Liu2, Wenbing Xu1, Jinmei Luo1, Jingwen Niu3, Zhi Liu3, Jinming Gao1, Jinglan Wang1, Yi Dai3, *( ), Mengzhao Wang1, *(
), Mengzhao Wang1, *( )
)
- 1Department of Respiratory and Critical Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
2Department of Endocrinology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
3Department of Neurology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
-
Received:2021-01-25Published:2021-12-31Online:2021-10-09 -
Contact:Yi Dai,Mengzhao Wang E-mail:pumchdy@pumch.cn;mengzhaowang@sina.com
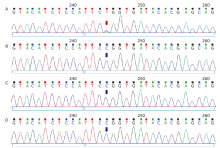
| In this report, the authors describe a sporadic case of a 14-year-old Chinese girl with a de novo p.Pro209Leu mutation in BAG3 who presented with skeletomuscular and peripheral nerve dysfunction in childhood and gradually appearing symptoms of hypercapnia that required assisted ventilation, suggesting that BAG3-associated MFM should be considered as a rare differential diagnosis of hypercapnia. |
Cite this article
Yan Xu, Shixuan Liu, Wenbing Xu, Jinmei Luo, Jingwen Niu, Zhi Liu, Jinming Gao, Jinglan Wang, Yi Dai, Mengzhao Wang. BAG3-Related Myofibrillar Myopathy Presenting as Hypercapnia: A Case Report and Literature Review[J].Chinese Medical Sciences Journal, 2021, 36(4): 265-278.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 1
Results of nerve conduction study"
| Nerve stimulated | Recording site | Distal or peak latency (ms) | Amplitude (mv) | Conduction velocity (m/s) |
|---|---|---|---|---|
| Medianus Motor Left | Wrist-APB | 3.91 (30%↑) | 10.5 | |
| Elbow-Wrist | 9.34 | 9.9 | 44.2 (32%↓) | |
| Medianus Motor Right | Wrist-APB | 3.96 (32%↑) | 7.1(68%↓) | |
| Elbow-Wrist | 8.96 | 5.8(68%↓) | 44.0 (32%↓) | |
| Ulnaris Motor Left | Wrist-ADM | 2.42 | 8.9 | |
| Above Elbow-Wrist | 8.34 | 8.2 | 43.1 (34%↓) | |
| Ulnaris Motor Right | Wrist-ADM | 2.6 | 8.3 | |
| Above Elbow-Wrist | 8.56 | 8.5 | 41.1 (37%↓) | |
| Tibialis Motor Left | Ankle-AH | NR | NR | |
| Peroneus Motor Left | Ankle-EDB | NR | NR | |
| Fibula Head-Ankle | NR | NR | ||
| Peroneus Motor Right | Ankle-EDB | NR | NR | NR |
| Fibula Head-Ankle | NR | NR | NR |
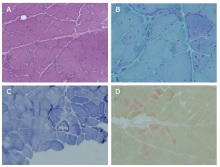
Figure 3.
Pathological evaluation of the left quadriceps femoris muscle. A. Hematoxylin/eosin staining showed transverse sections, with greater variability in muscle-fiber size and some scattered angular muscle fibers (×200). Several degenerated fibers are seen, and a few fibers contained cytoplasmic aggregates. There was no peri-fascicular atrophy, necrosis, or phagocytosis. B. Modified Gomori trichrome staining shows dark stained cytoplasmic aggregates in some fibers (×400). C. Nicotinamide adenine dinucleotide staining indicates irregular internal architecture in a few muscle fibers (×200). D. Acid phosphatase staining reveals deep stained positive particles in a few individual muscle fibers (×200)."

Table 2
Summary of the characteristics of the current patient and of the patients in the literature review"
| Patient number | References | Sex (age at onset) | Symptoms at onset | Skeletal myopathy | Other features | CK levels | Electromyography | Neuropathy | Electroneuro- graphy | Respiratory function | Cardiomyopathy [QTc (ms)] | Treatment | Parents | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
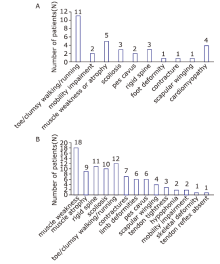
| 1 | Kostera-Pruszczyk et al.[ | Female (8 years old) | Toe-walking and foot deformity | Subclinical | Rigidity of cervical and thoracic spine, contractures at hips, knees, and ankles; bilateral pes cavus deformity, and absence of deep tendon reflexes in the lower extremities | 1.5-fold above normal | NR | Axonal-demyelinating sensory-motor polyneuropathy | Conduction velocity in the median/ulnar motor nerves, 38 m/s | Normal (FVC 87%) | Restrictive cardiomyopathy (mean 478 ms, max 574 ms) | NR | Unaffected | NR |
| 2 | Jaffer et al. [ | Female (1.5 years old) | Toe-walking | Proximodistal weakness, proximal muscle atrophy, and weakness of neck flexion | Rigid spine, Achilles tendon tightness, elbow/finger flexion contractures | NR | Chronic neurogenic changes, absent responses on stimulation of the phrenic nerves, and chronic denervation of the diaphragm | Giant axonal neuropathy (neuropathological axonal loss and giant thinly myelinated axons) | Some intermediate slowing of conduction velocity | NR | Restrictive cardiomyopathy (NR) | Heart transplantation at 13 years of age | Unaffected | NR |
| 3 | Jaffer et al. [ | Male (early childhood) | Toe-walking and high steppage gait | Proximal and distal muscle weakness and atrophy | Rigid spine, pectus carinatum, distal contractures and scoliosis | NR | Chronic neurogenic changes | Giant axonal neuropathy (giant thinly myelinated axons) | Some intermediate slowing of conduction velocity | FVC 63%→53%; nocturnal hypoventilation | Restrictive cardiomyopathy (NR) | Non-invasive ventilatory support | Unaffected | NR |
| 4 | Jaffer et al. [ | Male (11 months) | Toe-walking, clumsy, and frequent falls | Severe proximal and distal weakness | Rigid spine, scoliosis, Achilles tendon tightness, and lower limb deformities | 1054-2500 IU/L | NR | NR | NR | FVC 50% when standing, 39% when lying | Restrictive cardiomyopathy (NR) | Nocturnal non-invasive ventilatory support after the 9th month | Unaffected | Died of cardiorespiratory failure at age 12 years and 9 months |
| 5 | Jaffer et al. [ | Female (first decade) | Toe-walking and progressive mobility impairment | Minimal distal upper limb weakness and proximal weakness of the hip extensors and adductors | Pectus carinatum, tight Achilles tendons, and scoliosis (Cobb angle 104 °) | 154 IU/L | Chronic neurogenic changes | Axonal neuropathy | Some intermediate slowing of conduction velocity | FVC 46% | Restrictive cardiomyopathy (NR) | Nocturnal non-invasive ventilatory support | Her older sister and father similarly affected | NR |
| 6(older sister of patient No. 5) | Jaffer et al. [ | Female (8 years old, | Bilateral pes cavus and progressively impaired mobility | NR | NR | NR | NR | NR | NR | Respiratory weakness | NR | Nocturnal non-invasive ventilatory support in her 20s | As above | NR |
| 7 | Odgerel et al. [ | Male (12 years old) | Neck, proximal muscles of upper and lower extremities involved, and cardiomyopathy | Generalized muscle weakness and atrophy | Skeletal deformity | Normal | Neurogenic/myopathic changes | Giant axonal neuropathy | Axonal neuropathy | Respiratory weakness at 15 years of age | Restrictive/ hypertrophic cardiomyopathy (NR) | Heart transplantation at 14 years of age; ventilator dependency at age 29 years | Unaffected | Alive |
| 8 | Odgerel et al. [ | Male (9 years old) | Proximal muscles of upper and lower extremities involved, and cardiomyopathy | Generalized muscle weakness and atrophy | NR | NR | NR | NR | NR | Respiratory weakness at 19 years of age | Restrictive/ hypertrophic cardiomyopathy (NR) | NR | Unaffected | Sudden death at 9 years |
| 9 | Odgerel et al. [ | Female (12 years old) | Neck and distal lower extremity muscle weakness, bilateral pes cavus, and slight dorsal scoliosis | Neck and distal muscle weakness | Bilateral pes cavus and slight dorsal scoliosis | 2-3 times above normal | Myopathic changes | Giant axonal neuropathy | Axonal neuropathy | Respiratory weakness at 20 years of age | Restrictive/ hypertrophic cardiomyopathy (NR) | NR | Unaffected | Died at the age of 20 years of cardiac and respiratory failure |
| 10 | Odgerel et al. [ | Male (5 years old) | Problem running | Proximal muscle weakness | NR | Normal | Neurogenic/myopathic changes | Giant axonal neuropathy | Axonal neuropathy | Respiratory weakness at 10 years of age | Restrictive/ hypertrophic cardiomyopathy (NR) | Heart transplantation at 13 years of age; ventilator dependent at 13 years of age | Unaffected | Died at 15 years of age |
| 11 | Lee et al. [ | Female (6 years old) | Progressive clumsy walking and easy falling during exercise | Mild proximal muscle weakness of both legs and later both arms | Rigid spine and scoliosis, contractures, and foot deformity | 991 IU/L (normal range < 154) | Increase in duration of the action potential with normal amplitude | Axonal neuropathy | Decreased motor (tibial and peroneal) amplitudes and latencies | Restrictive lung disease | Restrictive/ hypertrophic cardiomyopathy (450-570 ms) | NR | Her father was a carrier of c.772C>T (p. Arg258 Trp) | NR |
| 12 | Konersman et al. [ | Male (8 years old) | Restrictive cardiomyopathy | Severe diffuse muscle atrophy and weakness (distal> proximal), and mild bilateral lower facial weakness | Toe walking, imbalance, foot deformity, spinal rigidity | 669 to 1099 IU/L (normal range: 55-170) | Fibrillation potentials in the proximal and distal muscles, giant motor unit potentials (> 10 mV) in the distal muscles, and reduced recruitment in all muscles | Sensorimotor polyneuropathy with mixed axonal and demyelinating features and indolent primarily axonal neuropathy with secondary demyelination/remyelination | Upper extremity motor conduction velocities were in the “intermediate” range | Difficulty breathing with hypercapnic respiratory insufficiency of neuromuscular etiology; intermittent ventilation during the day and continuous at night | Restrictive cardiomyopathy | Orthotopic heart transplant at 8 years of age, intermittent ventilation throughout the day and continuous ventilation at night via tracheostomy | Unaffected | NR |
| 13 | Noury et al.[ | Female (10 years old) | Inability to run and maintain prolonged standing | Proximal and distal lower limb weakness; neck flexion was impossible | Rigid spine, varus foot deformity, bilateral scapular winging, and hypophonia | Normal | Mild myogenic changes in the upper limb proximal muscles | Severe sensory-motor axonal neuropathy predominant in the lower limbs with mild myogenic changes in the upper limb proximal muscles | NR | FVC 42% | Normal (NR) | No | NR | NR |
| 14 | Selcen et al.[ | Male (early childhood) | Toe-walking | Severe diffuse muscle weakness and atrophy | Contractures at the knees and ankles and bilateral diaphragm paralysis | 3-fold above normal | NR | NR | NR | Respiratory insufficiency | Restrictive cardiomyopathy (NR) | Heart transplantation at 13 years of age | NR | NR |
| 15 | Selcen et al.[ | Female (13 years old) | Scoliosis, rigid spine, and easy fatigability | Axial, moderately severe proximal and distal muscle weakness | Spinal stiffness, and hypernasal speech | 6-fold above normal | Myopathic motor unit potentials | Axonal and demyelinating peripheral neuropathy | Axonal and demyelinating peripheral neuropathy | Restrictive respiratory insufficiency | Hypertrophic cardiomyopathy (NR) | Nocturnal non-invasive ventilatory support | NR | NR |
| 16 | Selcen et al.[ | Male (7 years old) | Toe-walking | Weakness of the axial and proximal limb muscles | Valgus ankle deformity, thoracic scoliosis, scapular winging, and rigid spine | 15-fold above normal | NR | NR | NR | Reduced FVC and respiratory insufficiency | Restrictive cardiomyopathy (NR) | Did not tolerate nocturnal ventilatory support | NR | Died following a chest infection |
| 17 | Schänzer et al.[ | Male (4 years old) | Restrictive cardiomyopathy | Proximal muscle weakness | Mild scoliosis | Slightly increased (227 IU/L; normal range < 180) | Myopathic pattern | Combined demyelinating and axonal polyneuropathy | Combined demyelinating and axonal polyneuropathy | VC 53% and nocturnal hypoxemia and hypercapnia | Restrictive cardiomyopathy | Heart transplantation, non-invasive ventilatory support | Unaffected | NR |
| 18 | D’Avila et al.[ | Female (11 years old to 14 years old) | Spinal contractures causing spinal rigidity, scapular winging, and later postural muscle atrophy | Postural muscle atrophy and proximal weakness in a limb girdle distribution pattern with loss of deambulation | Spinal contractures causing spinal rigidity, scapular winging | Normal to 1.500 U/L | Axonal neuropathy | Axonal neuropathy | NR | Respiratory insufficiency | Impaired conduction, arrhythmia, and cardiac hypertrophy | Ventilatory support on a continuous basis or overnight. | Unaffected | NR |
| 19 | Latham et al.[ | Male (2 years old) | Toe walking and progressive decreased range of motion of the neck and lower extremities | Loss of gross and fine motor control | Severe contractures of the lower extremities, scoliosis, rigid spine, and neck extension contracture | 110 to 510 IU/L (normal range: 35-230) | NR | NR | NR | Obstructive sleep apnea, and severe restrictive lung disease | Restrictive cardiomyopathy | Cardiac transplantation, nighttime noninvasive ventilation | NR | NR |
| 20 | Semmler et al.[ | Male (34 years old) | Distal lower limb weakness and symmetrical calve atrophy | Skeletal muscle weakness | Scapular winging | 1050 IU/L | Mixed pattern | Axonal sensorimotor polyneuropathy | NR | Normal | Normal | NR | NR | NR |
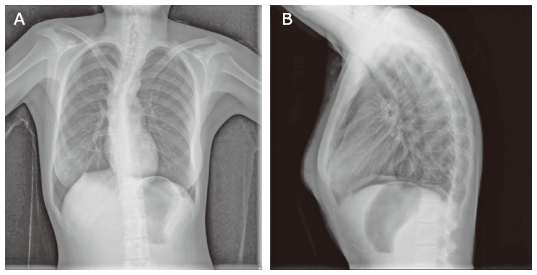
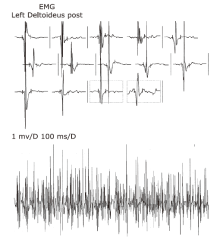
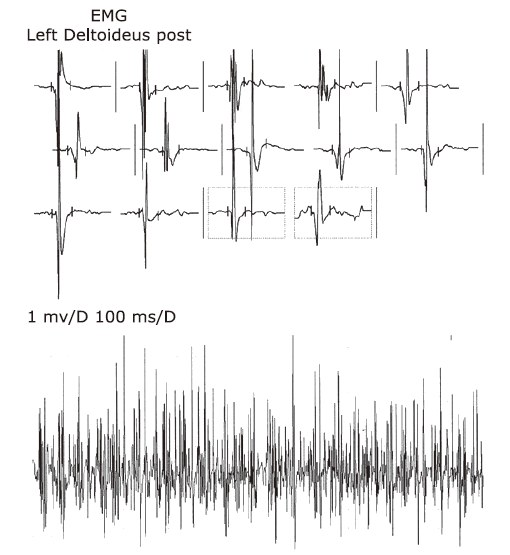
| 21 | Current patient | Female (14 years old) | Scoliosis | Axial, both upper and lower extremity muscle weakness and atrophy | Clumsy walking, difficulty in squatting, unstable gait | 2-fold above normal | Neurogenic damage pattern combined with evidence of myogenic damage | Demyelinating and axonal neuropathy | Both motor and sensory nerves were impaired; the condition of the nerves in the lower extremities was more severe than that of nerves of the upper extremities and there was no wave on the peroneus nerve test | Severe restrictive ventilation dysfunction | Normal (461 ms) | Non-invasive ventilatory support | Unaffected | Alive |
Table 3
Summary of the histology of the muscle biopsy of the current patient and of the patients in the literature review"
| Patient number | Histology of the muscle biopsy |
|---|---|
| 1 | Large deposits in fibers, Z-disc streaming, disorganization of myofibrils, and sarcomeric disorganization with accumulation of polymorphic dense structures; focal changes containing granulomatous material. |
| 2 | NR |
| 3-4 | Accumulation of myotilin and desmin, small atrophic fibers consistent with denervation, granulomatous material, myofibrillar loss, Z-line streaming. |
| 5 | NR |
| 6 | NR |
| 7-8 | Myofibrillar breakdown, presence of desmin-reactive inclusions, Z-line streaming. |
| 9 | Accumulation of dark dense material, increment of intermyofibrillar spaces, and multiple core areas, desmin positive inclusions, electron dense filamentous and granular material disrupting the Z-lines |
| 10 | Atrophic fibers, focal myofibrillar disorganization, and dark inclusions with prominence of desmin |
| 11 | Atrophic fibers, focal myofibrillar disorganization, accumulation of electron-dense granulofilamentous materials, myofibrillar degeneration with minicores |
| 12 | Fiber size variation with atrophic and hypertrophic fibers, myofibrillar disruption, scattered fibers with inclusions and vacuoles |
| 13 | Accumulation of granulofilamentous material originating from the Z-disk disrupting the sarcomeres, accumulation of dense granulomatous material |
| 14-16 | Fiber size variation, protein accumulation, Z disk streaming and accumulation of electron dense structures, disarray of myofibrils, mitochondrial cluster, and apoptotic nuclei |
| 17 | Myofibrillar disintegration, intracytoplasmic inclusions containing different proteins, myofibrillar disintegration, and Z-disk streaming |
| 18 | Z disk aggregates and atrophic type I fibers, whereas typeⅡfibers were hypertrophic |
| 19 | Myofibril dissolution and accumulation of degradation products at the Z-disk |
| 20 | Z-disk streaming and accumulation of granulofilamentous material |
| 21 | Variability in muscle-fiber size, some scattered angular muscle fibers and several degenerated fibres. Dark stained cytoplasmic, irregular internal architecture, deep stained positive particles could be seen in fibers. |
| 1. |
Selcen D. Myofibrillar myopathies. Neuromuscul Disord 2011; 21(3):161-71. doi: 10.1016/j.nmd.2010.12.007.
doi: 10.1016/j.nmd.2010.12.007 |
| 2. |
Semmler AL, Sacconi S, Bach JE, et al. Unusual multisystemic involvement and a novel BAG3 mutation revealed by NGS screening in a large cohort of myofibrillar myopathies. Orphanet J Rare Dis 2014; 9:121. doi: 10.1186/s13023-014-0121-9.
doi: 10.1186/s13023-014-0121-9 |
| 3. |
D’Avila F, Meregalli M, Lupoli S, et al. Exome sequencing identifies variants in two genes encoding the LIM-proteins NRAP and FHL1 in an Italian patient with BAG3 myofibrillar myopathy. J Muscle Res Cell Motil 2016; 37(3):101-15. doi: 10.1007/s10974-016-9451-7.
doi: 10.1007/s10974-016-9451-7 |
| 4. |
Batonnet-Pichon S, Behin A, Cabet E, et al. Myofibrillar myopathies: new perspectives from animal models to potential therapeutic approaches. J Neuromuscul Dis 2017; 4(1):1-15. doi: 10.3233/JND-160203.
doi: 10.3233/JND-160203 pmid: 28269794 |
| 5. |
Rauch JN, Tse E, Freilich R, et al. BAG3 is a modular, scaffolding protein that physically links heat shock protein 70 (Hsp70) to the small heat shock proteins. J Mol Biol 2017; 429(1):128-41. doi: 10.1016/j.jmb.2016.11.013.
doi: 10.1016/j.jmb.2016.11.013 |
| 6. |
Rosati A, Graziano V, De Laurenzi V, et al. BAG3: a multifaceted protein that regulates major cell pathways. Cell Death Dis 2011; 2(4):e141. doi: 10.1038/cddis.2011.24.
doi: 10.1038/cddis.2011.24 |
| 7. |
Dirk B, Stefan R, Claudia SG, et al. Tuning of chaperone activity of Hsp70 proteins by modulation of nucleotide exchange. Nat Struct Biol 2001; 8(5):427-32. doi: 10.1038/87588.
doi: 10.1038/87588 |
| 8. |
Selcen D, Muntoni F, Burton BK, et al. Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann Neurol 2008; 65(1):83-9. doi: 10.1002/ana.21553.
doi: 10.1002/ana.21553 |
| 9. |
Lee HC, Cherk SW, Chan SK, et al. BAG3-related myofibrillar myopathy in a Chinese family. Clin Genet 2012; 81(4):394-8. doi: 10.1111/j.1399-0004.2011.01659.x.
doi: 10.1111/j.1399-0004.2011.01659.x pmid: 21361913 |
| 10. |
Odgerel Z, Lee HS, McKenna C, et al. Inheritance patterns and phenotypic features of myofibrillar myopathy associated with a BAG3 mutation. Neuromuscul Disord 2010; 20(7):438-42. doi: 10.1016/j.nmd.2010.05.004.
doi: 10.1016/j.nmd.2010.05.004 |
| 11. |
Jaffer F, Scoto M, Healy E, et al. BAG3 mutations: another cause of giant axonal neuropathy. J Peripher Nerv Syst 2012; 17(2):210-6. doi: 10.1111/j.1529-8027.2012.00409.x.
doi: 10.1111/j.1529-8027.2012.00409.x |
| 12. |
Noury JB, Maisonobe T, Richard P, et al. Rigid spine syndrome associated with sensory-motor axonal neuropathy resembling Charcot-Marie-Tooth disease is characteristic of Bcl-2-associated athanogene-3 gene mutations even without cardiac involvement. Muscle Nerve 2018; 57(2):330-4. doi: 10.1002/mus.25631.
doi: 10.1002/mus.25631 |
| 13. |
Kostera-Pruszczyk A, Suszek M, Ploski R, et al. BAG3-related myopathy, polyneuropathy and cardiomyopathy with long QT syndrome. J Muscle Res Cell Motil 2015; 36(6):423-32. doi: 10.1007/s10974-015-9431-3.
doi: 10.1007/s10974-015-9431-3 |
| 14. |
Konersman CG, Bordini BJ, Scharer G, et al. BAG3 myofibrillar myopathy presenting with cardiomyopathy. Neuromuscul Disord 2015; 25(5):418-22. doi: 10.1016/j.nmd.2015.01.009.
doi: 10.1016/j.nmd.2015.01.009 |
| 15. |
Schänzer A, Rupp S, Gräf S, et al. Dysregulated autophagy in restrictive cardiomyopathy due to Pro209Leu mutation in BAG3. Molecul Genet Metab 2018; 123(3):388-99. doi: 10.1016/j.ymgme.2018.01.001.
doi: 10.1016/j.ymgme.2018.01.001 |
| 16. |
Latham GJ, Lopez G. Anesthetic considerations in myofibrillar myopathy. Paediatr Anaesth 2015; 25(3):231-8. doi: 10.1111/pan.12516.
doi: 10.1111/pan.12516 |
| 17. |
Olive M, Odgerel Z, Martinez A, et al. Clinical and myopathological evaluation of early- and late-onset subtypes of myofibrillar myopathy. Neuromuscul Disord 2011; 21(8):533-42. doi: 10.1016/j.nmd.2011.05.002.
doi: 10.1016/j.nmd.2011.05.002 |
| 18. |
Olivé M, Kley RA, Goldfarb LG. Myofibrillar myopathies: new developments. Curr Opin Neurol 2013; 26(5):527-35. doi: 10.1097/WCO.0b013e328364d6b1.
doi: 10.1097/WCO.0b013e328364d6b1 |
| 19. |
Naddaf E, Milone M. Hereditary myopathies with early respiratory insufficiency in adults. Muscle Nerve 2017; 56(5):881-6. doi: 10.1002/mus.25602.
doi: 10.1002/mus.25602 pmid: 28181274 |
| 20. |
Homma S, Iwasaki M, Shelton GD, et al. BAG3 deficiency results in fulminant myopathy and early lethality. Am J Pathol 2006; 169(3):761-73. doi: 10.2353/ajpath.2006.060250.
doi: 10.2353/ajpath.2006.060250 |
| 21. |
Jackson S, Schaefer J, Meinhardt M, et al. Mitochondrial abnormalities in the myofibrillar myopathies. Eur J Neurol 2015; 22(11):1429-35. doi: 10.1111/ene.12814.
doi: 10.1111/ene.12814 pmid: 26204918 |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|