Chinese Medical Sciences Journal ›› 2022, Vol. 37 ›› Issue (1): 1-14.doi: 10.24920/003954
• Original Article • Next Articles
Minocycline Activates the Nucleus of the Solitary Tract-Associated Network to Alleviate Lipopolysaccharide-Induced Neuroinflammation
Jianbo Xiu1, 2, Lanlan Li1, 2, Qi Xu1, 2, *( )
)
- 1State Key Laboratory of Medical Molecular Biology, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, School of Basic Medicine Peking Union Medical College, Beijing 100005, China
2Neuroscience Center, Chinese Academy of Medical Sciences, Beijing 100005, China
-
Received:2021-06-10Accepted:2021-06-29Published:2022-03-31Online:2021-06-30 -
Contact:Qi Xu E-mail:xuqi@pumc.edu.cn
| Minocycline is a tetracycline derivative with good permeability through blood-brain barrier and exerts a variety of neuroprotective effects by inhibiting microglia activation. However, how minocycline exerts its effects across the whole brain remains unclear. The authors examined the Lipopolysaccharide (LPS)-induced activation of microglia and expression of c-Fos by immunohistochemistry throughout the mouse brain at 6 hours and 24 hours after treatment. They also investigated with minocycline pretreatment to identify which brain regions can suppress activation of microglia and which brain network may be involved in the antidepressant effects of minocycline. It was found that minocycline may attenuate LPS-induced neuroinflammation by activating the NTS-associated network. |
Cite this article
Jianbo Xiu, Lanlan Li, Qi Xu. Minocycline Activates the Nucleus of the Solitary Tract-Associated Network to Alleviate Lipopolysaccharide-Induced Neuroinflammation[J].Chinese Medical Sciences Journal, 2022, 37(1): 1-14.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 1
Statistical summary of the cell body area of microglia (μm2) in various regions across the brains of mice treated with saline(S)/minocycline(M) and saline/LPS(L)§"
| Brain Region | number of microglia cells | cell body areas of microglia (μm2) of the group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| S3S6 | S3L6 | M3S6 | M3L6 | S3S24 | S3L24 | M3S24 | M3L24 | ||
| Acbs | 850 | 29.5±1.4 | 48.3±2.8††† | 33.8±1.7 | 34.7±1.8*** | 31.0±1.7 | 31.2±1.7 | 34.2±1.6 | 41.2±1.9### |
| BNSTd | 1077 | 31.3±1.6 | 41.2±2.2†† | 37.3±2.0 | 35.7±2.3 | 31.9±1.9 | 40.0±2.3 | 35.7±2.0 | 49.7±2.8## |
| PVN | 698 | 28.8±1.4 | 43.0±4.1††† | 29.7±1.4 | 32.6±3.1* | 30.5±1.3 | 38.5±2.4‡ | 32.9±1.5 | 40.8±2.4 |
| CeA | 1461 | 32.9±1.8 | 49.4±2.2††† | 38.0±1.2 | 39.8±2.7*** | 32.4±1.2 | 39.5±1.7‡‡ | 37.6±1.3 | 47.5±2.0### |
| DG | 1405 | 35.7±1.4 | 49.9±2.5††† | 38.1±2.0 | 44.7±2.2 | 34.7±1.4 | 49.4±2.3‡‡ | 39.9±1.5 | 45.6±2.0 |
| LPB | 846 | 33.9±1.9 | 44.9±3.1†† | 35.4±1.6 | 41.0±2.6 | 30.0±1.5 | 43±2.2‡‡‡ | 35.4±1.6 | 38.3±2.2 |
| LC | 590 | 31.7±1.5 | 43.8±3.2††† | 36.6±1.8 | 37.2±2.0* | 32.8±1.6 | 38.7±1.9‡ | 35.6±3.9 | 36.8±2.9 |
| NTS | 927 | 30.8±1.7 | 50.7±3.1††† | 37.2±2.2 | 35.9±2.1*** | 33.2±1.9 | 38.9±2.1 | 35.1±1.9 | 40.8±2.4 |
| AP | 1061 | 46.2±4.5 | 54.8±3.2 | 55.3±3.3 | 58.5±4.8 | 48.9±3.2 | 57.9±2.5 | 47.0±3.5 | 54.2±3.2 |
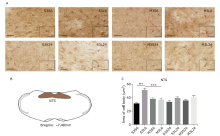
Figure 2.
Microglial activation in nucleus tractus solitarius (NTS) in response to lipopolysaccharide (LPS) and minocycline treatment. (A) Microglia morphology detected by Iba-1 immunohistochemistry in NTS of mice of the eight groups. Scale bars: 60μm; scale bars of the insert: 30μm. (B) Schematic illustration of the area of NTS analyzed in this study. (C) Statistical summary of the cell body area of microglia (μm2) in NTS of different groups of animals. Values were expressed as the mean ± SEM. S3S6: n = 97; S3L6: n = 123; M3S6: n = 133; M3L6: n = 75; S3S24: n = 107; S3L24: n = 124; M3S24: n = 118; M3L24: n = 150. †††P<0.001; ***P<0.001."
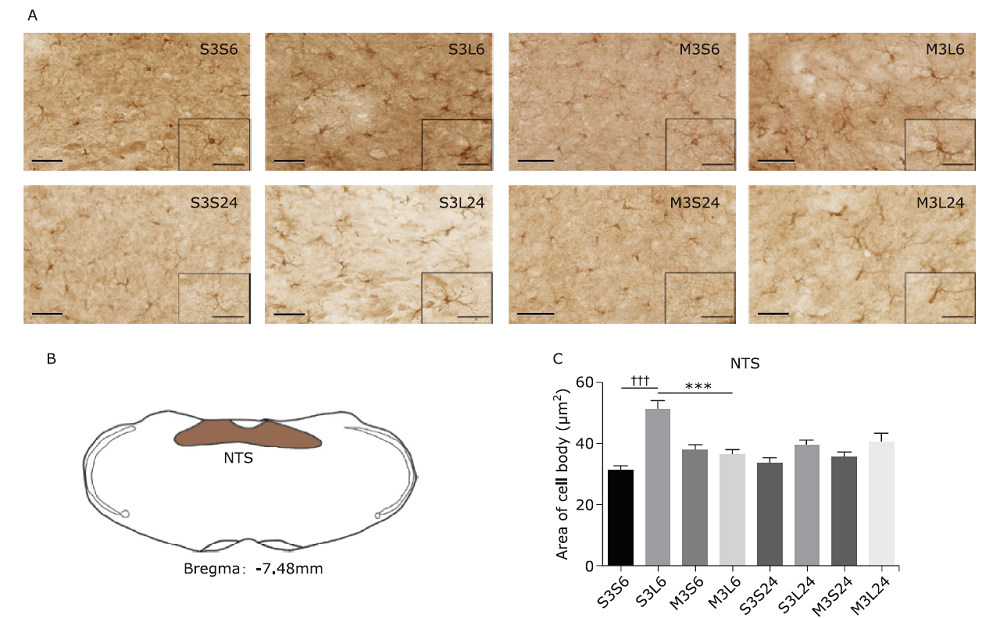
Table 2
Statistical summary of the number of c-Fos immunoreactive neurons in various regions across the brains of mice treated with saline(s)/minocycline(M) and saline/LPS(L)§"
| Brain Region | number of c-Fos immunocreative neurons of the group (n) | |||||||
|---|---|---|---|---|---|---|---|---|
| S3S6 | S3L6 | M3S6 | M3L6 | S3S24 | S3L24 | M3S24 | M3L24 | |
| Acbc | 0 | 0 | 0 | 0 | 0 | 35.4±6.8‡‡‡ | 0 | 30.2± 2.5 |
| Acbs | 0 | 37.8±2.5† | 8.6±2.2 | 23.0±5.7 | 0 | 59.0±11.9 | 41.8±15.7 | 87.6±18.1 |
| BNSTd | 0 | 29.0±4.9††† | 4.4±2.9 | 68.3±11.6*** | 0 | 7.2±2.8‡‡ | 4.4±2.77 | 12.4±3.9 |
| BNSTv | 5.0±3.5 | 46.0±7.3††† | 2±2 | 123.0±17.2*** | 0 | 16.8±2.4‡‡ | 5.8±2.7 | 27.4±8.3# |
| PVN | 0.8±0.8 | 174±19.8††† | 6±1.1 | 240.0±3.6*** | 15.4±7.4 | 36.4±6.2‡‡‡ | 17.2±4.3 | 90.8±21.9## |
| CeA | 0 | 28.0±3††† | 6.8±2 | 52.6±7.8*** | 2.0±2.0 | 13.6±4.2‡ | 11.6±3.2 | 45.0±5.8### |
| BLA | 0 | 25.3±2††† | 6±0.8 | 9.4±3.0* | 3.2±3.2 | 7.8±3.4‡ | 9.8±4.7 | 47.2±9.3### |
| DG | 0 | 0 | 0 | 0 | 5.0±1.4 | 39.4±6.8‡‡‡ | 16.2±4.3 | 36.2±2.5 |
| VTA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DR | 1.0±1.0 | 65.0±7.9†† | 12.4±3.5 | 121.6±21.0** | 11.8±8.9 | 60.4±19.6 | 29.2±4.1 | 83.4±10.6 |
| PAG | 7.0±1.2 | 14.0±1.8 | 9.0±1.9 | 51.0±8.3*** | 8.0±5.8 | 18±5.7 | 25.4±6.7 | 103.0±9.5### |
| LPB | 3.0±1.8 | 36.0±12††† | 5.8±2.9 | 101.0±8.5*** | 2.0±2.0 | 21.0±4.3 | 1.8±1.1 | 38.0±5.6# |
| LC | 4.3±3.3 | 34.0±2.1† | 6.4±4.7 | 70.4±8.1** | 14.6±9.0 | 24.6±5.9 | 52.8±7.4 | 58.2±10.8## |
| NTS | 0 | 104.8±8.2††† | 0 | 204.8±13.5*** | 13.8±7.8 | 106.8±35.9 | 11.6±1.3 | 97.4±9.4 |
| AP | 0 | 72.0±8.6††† | 0 | 170.6±22.1*** | 0 | 4.3±4.3‡‡‡ | 0 | 3.0±2.1 |
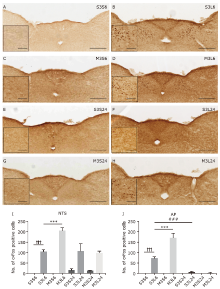
Figure 3.
Neuronal activation in nucleus tractus solitarius (NTS) and area postrema (AP) in response to LPS and minocycline treatment. (A-H) Representative images showing c-Fos immunostaining. Scale bars: 100 μm. scale bar of the insert: 25 μm (I, J) Statistical summary of the number of c-fos positive neurons in NTS and AP. Values were expressed as mean ± SEM. n = 4-5 per group, n = 37 per brain region. †††P<0.001, ***P<0.001, ###P<0.001."
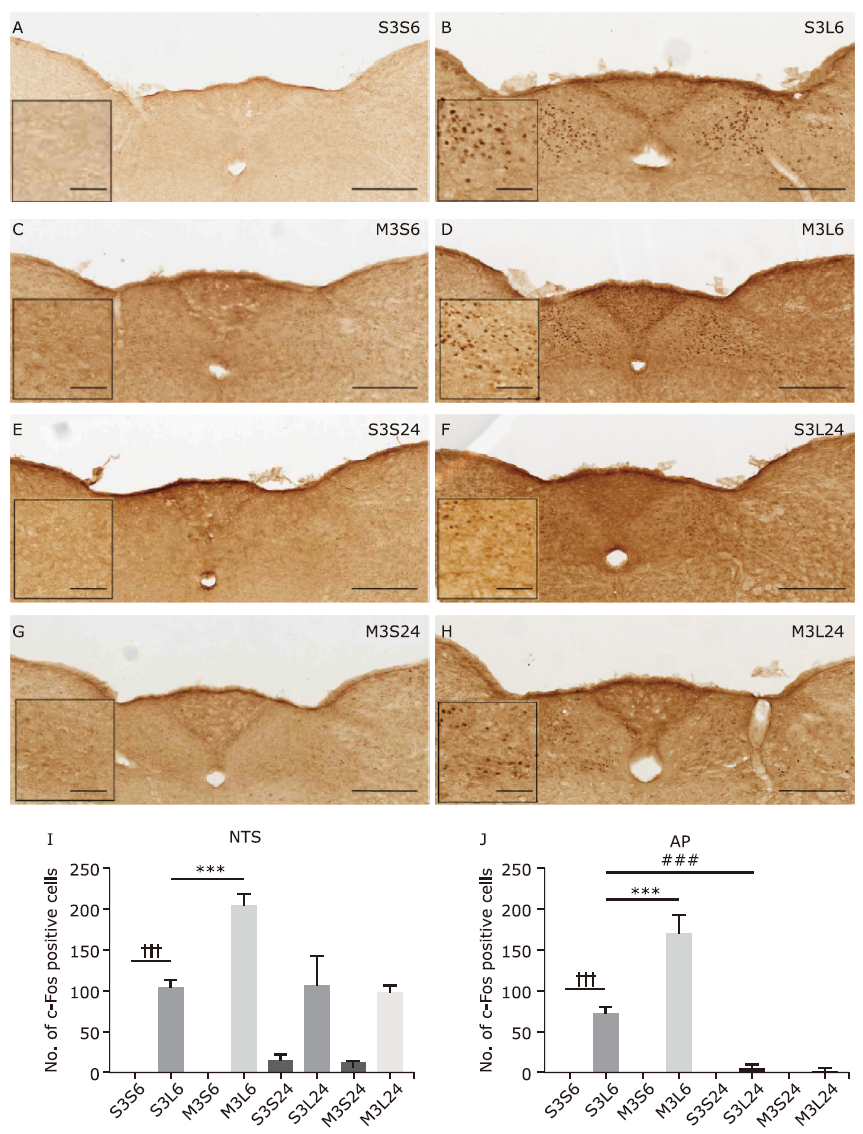
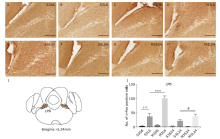
Figure 4.
Neuronal activation in lateral parabrachial nucleus (LPB) in response to LPS and minocycline treatment. (A-H) Representative images showing c-Fos immunostaining. Scale bars: 100 μm. (I) Schematic illustration of the area of LPB analyzed in this study. (J) Statistical summary of the number of c-Fos positive neurons. Values are expressed as mean ± SEM. n = 4-5 per group, n = 38 per brain region. †††P<0.001; ***P<0.001; #P<0.05."

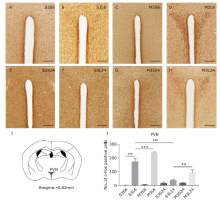
Figure 5.
Neuronal activation in paraventricular nucleus (PVN) in response to LPS and minocycline treatment. (A-H) Representative images showing c-Fos immunostaining. Scale bars: 100 μm. (I) Schematic illustration of the area of PVN analyzed in this study.(J) Statistical summary of the number of c-Fos positive neurons in PVN. Values are expressed as mean ± SEM. n = 4-5 per group, n = 38 per brain region. †††P<0.001; ***P<0.001; ‡‡‡ P<0.001, ##P<0.01."
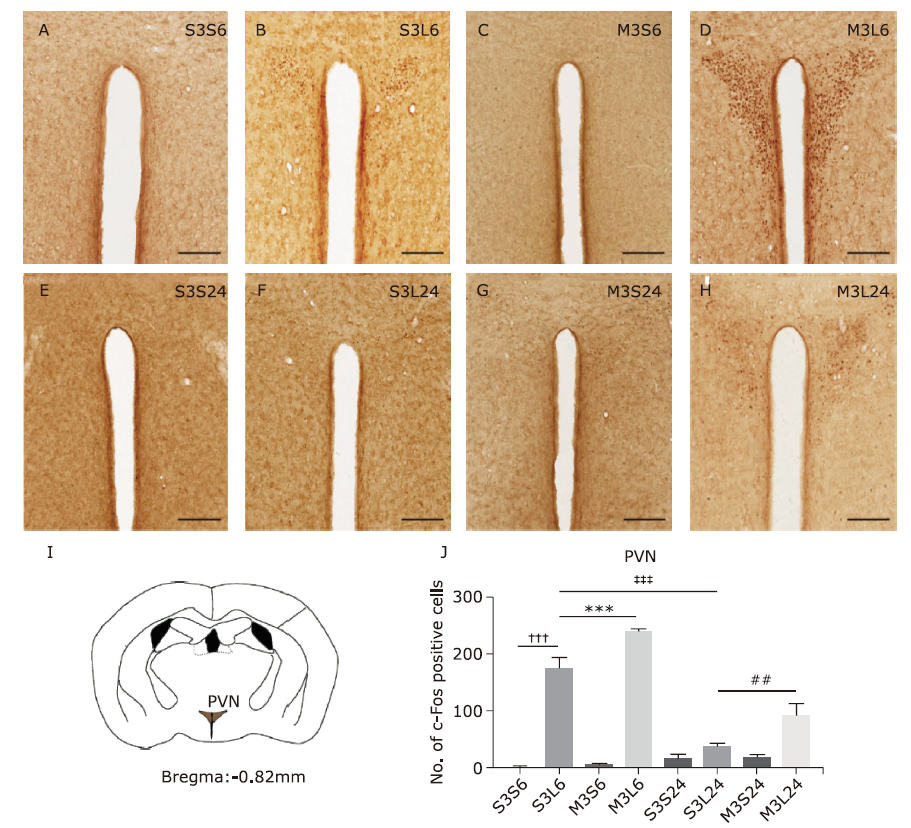
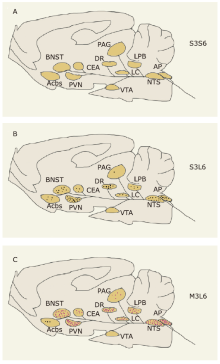
Figure 6.
Schematic summary of neuronal activation in the NTS-associated network in response to lipopolysaccharide (LPS) and minocycline. (A) Neuronal activation in the brains of animals treated with saline for three days and once more 6 hours before sacrifice (group S3S6). (B) Neuronal activation in the brains of animals treated with saline for three days and LPS for 6 hours before sacrifice (group S3L6). (C) Neuronal activation in the brains of animals treated with minocycline for three days and LPS for 6 hours before sacrifice (group M3L6). Brain regions with red dots show significantly increased number of c-Fos-positive neurons in group M3L6 compared with group S3L6. Each dot in every brain region represents one-tenth of the actual number of c-Fos positive neurons."

| 1. |
Wohleb ES, Franklin T, Iwata M, et al. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci 2016; 17(8):497-511. doi: 10.1038/nrn.2016.69.
doi: 10.1038/nrn.2016.69 pmid: 27277867 |
| 2. |
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2016(1): 16, 22-34. doi: 10.1038/nri.2015.5.
doi: 10.1038/nri.2015.5 |
| 3. |
Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9(1):46-56. doi: 10.1038/nrn2297.
doi: 10.1038/nrn2297 pmid: 18073775 |
| 4. |
Hodes GE, Kana V, Menard C, et al. Neuroimmune mechanisms of depression. Nat neurosci 2015; 18(10):1386-93. doi: 10.1038/nn.4113.
doi: 10.1038/nn.4113 |
| 5. |
Beurel E, Toups M, Nemeroff CB. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020; 107(2):234-56. doi: 10.1016/j.neuron.2020.06.002.
doi: 10.1016/j.neuron.2020.06.002 |
| 6. |
Zhan L, Krabbe G, Du F, et al. Proximal recolonization by self-renewing microglia re-establishes microglial homeostasis in the adult mouse brain. PLoS biology 2019; 17(2):e3000134. doi: 10.1371/journal.pbio.3000134.
doi: 10.1371/journal.pbio.3000134 |
| 7. |
Lawson LJ, Perry VH, Dri P, et al. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neurosci 1990; 39(1):151-70. doi: 10.1016/0306-4522(90)90229-w.
doi: 10.1016/0306-4522(90)90229-w |
| 8. |
Prinz M, Jung S, Priller J. Microglia Biology: One Century of Evolving Concepts. Cell 2019; 179(2):292-311. doi: 10.1016/j.cell.2019.08.053.
doi: 10.1016/j.cell.2019.08.053 |
| 9. |
Deng SL, Chen JG, Wang F. Microglia: A central player in depression. Curr Med Sci 2020; 40(3):391-400. doi: 10.1007/s11596-020-2193-1.
doi: 10.1007/s11596-020-2193-1 |
| 10. |
Jia X, Gao Z, Hu H. Microglia in depression: current perspectives. Sci China Life Sci 2021; 64(6):911-25. doi: 10.1007/s11427-020-1815-6.
doi: 10.1007/s11427-020-1815-6 |
| 11. |
Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci 2015; 38(10):637-58. doi: 10.1016/j.tins.2015.08.001.
doi: S0166-2236(15)00176-9 pmid: 26442697 |
| 12. |
Dantzer R. Cytokine-induced sickness behavior: Mechanisms and implications. Ann Ny Acad Sci 2001; 933, 222-34. doi: 10.1111/j.1749-6632.2001.tb05827.x.
doi: 10.1111/j.1749-6632.2001.tb05827.x pmid: 12000023 |
| 13. |
Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun 2007; 21(6):727-35. doi: 10.1016/j.bbi.2007.05.005.
doi: 10.1016/j.bbi.2007.05.005 |
| 14. |
Henry CJ, Huang Y, Wynne A, et al. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation 2008; 5:15. doi: 10.1186/1742-2094-5-15.
doi: 10.1186/1742-2094-5-15 |
| 15. |
Soczynska JK, Mansur RB, Brietzke E, et al. Novel therapeutic targets in depression: Minocycline as a candidate treatment. Behav Brain Res 2012; 235(2):302-17. doi: 10.1016/j.bbr.2012.07.026.
doi: 10.1016/j.bbr.2012.07.026 pmid: 22963995 |
| 16. |
Zhu FR, Zheng YJ, Ding YQ, et al. Minocycline and Risperidone Prevent microglia activation and rescue behavioral deficits induced by neonatal intrahippocampal injection of lipopolysaccharide in rats. Plos One 2014; 9(4):e93966. doi: 10.1371/journal.pone.0093966.
doi: 10.1371/journal.pone.0093966 |
| 17. |
Zheng LS, Kaneko N, Sawamoto K. Minocycline treatment ameliorates interferon-alpha-induced neurogenic defects and depression-like behaviors in mice. Front Cell Neurosci 2015; 9:5. doi: 10.3389/fncel.2015.00005.
doi: 10.3389/fncel.2015.00005 |
| 18. |
Majidi J, Kosari-Nasab M, Salari AA. Developmental minocycline treatment reverses the effects of neonatal immune activation on anxiety- and depression-like behaviors, hippocampal inflammation, and HPA axis activity in adult mice. Brain Res Bull 2016; 120:1-13. doi: 10.1016/j.brainresbull.2015.10.009.
doi: 10.1016/j.brainresbull.2015.10.009 pmid: 26521068 |
| 19. |
Husain MI, Chaudhry IB, Husain N, et al. Minocycline as an adjunct for treatment-resistant depressive symptoms: A pilot randomised placebo-controlled trial. J Psychopharmacol 2017; 31(9):1166-75. doi: 10.1177/0269881117724352.
doi: 10.1177/0269881117724352 |
| 20. |
Han Y, Zhang LJ, Wang QZ, et al. Minocycline inhibits microglial activation and alleviates depressive-like behaviors in male adolescent mice subjected to maternal separation. Psychoneuroendocrinology 2019; 107:37-45. doi: 10.1016/j.psyneuen.2019.04.021.
doi: 10.1016/j.psyneuen.2019.04.021 |
| 21. |
Zhang C, Zhang YP, Li YY, et al. Minocycline ameliorates depressive behaviors and neuro-immune dysfunction induced by chronic unpredictable mild stress in the rat. Behav Brain Res 2019; 356:348-57. doi: 10.1016/j.bbr.2018.07.001.
doi: S0166-4328(18)30395-4 pmid: 30003978 |
| 22. |
Wang B, Huang X, Pan X, et al. Minocycline prevents the depressive-like behavior through inhibiting the release of HMGB1 from microglia and neurons. Brain Behav Immun 2020; 88:132-43. doi: 10.1016/j.bbi.2020.06.019.
doi: S0889-1591(20)30334-2 pmid: 32553784 |
| 23. |
Yang Q, Luo L, Sun T, et al. Chronic minocycline treatment exerts antidepressant effect, inhibits neuroinflammation, and modulates gut microbiota in mice. Psychopharmacology 2020; 237:3201-13. doi: 10.1007/s00213-020-05604-x.
doi: 10.1007/s00213-020-05604-x pmid: 32671421 |
| 24. |
Nettis MA, Lombardo G, Hastings C, et al. Augmentation therapy with minocycline in treatment-resistant depression patients with low-grade peripheral inflammation: results from a double-blind randomised clinical trial. Neuropsychopharmacology 2021; 46(5):939-48. doi: 10.1038/s41386-020-00948-6.
doi: 10.1038/s41386-020-00948-6 |
| 25. |
Bassett B, Subramaniyam S, Fan Y, et al. Minocycline alleviates depression-like symptoms by rescuing decrease in neurogenesis in dorsal hippocampus via blocking microglia activation/phagocytosis. Brain Behav Immun 2021; 91:519-30. doi: 10.1016/j.bbi.2020.11.009.
doi: 10.1016/j.bbi.2020.11.009 pmid: 33176182 |
| 26. |
Rosenblat JD, McIntyre RS. Efficacy and tolerability of minocycline for depression: a systematic review and meta-analysis of clinical trials. J Affect Disorders 2018; 227:219-25. doi: 10.1016/j.jad.2017.10.042.
doi: S0165-0327(17)31998-5 pmid: 29102836 |
| 27. |
Reis DJ, Casteen EJ, Ilardi SS. The antidepressant impact of minocycline in rodents: a systematic review and meta-analysis. Sci Rep 2019; 9(1):261. doi: 10.1038/s41598-018-36507-9.
doi: 10.1038/s41598-018-36507-9 |
| 28. |
Burke NN, Kerr DM, Moriarty O, et al. Minocycline modulates neuropathic pain behaviour and cortical M1-M2 microglial gene expression in a rat model of depression. Brain Behav Immun 2014; 42:147-56. doi: 10.1016/j.bbi.2014.06.015.
doi: 10.1016/j.bbi.2014.06.015 |
| 29. |
Yrjanheikki J, Keinanen R, Pellikka M, et al. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. P Natl Acad Sci U S A 1998; 95(26):15769-74. doi: 10.1073/pnas.95.26.15769.
doi: 10.1073/pnas.95.26.15769 |
| 30. |
Tikka T, Fiebich BL, Goldsteins G, et al. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci 2001; 21(8):2580-8. doi: 10.1523/Jneurosci.21-08-02580.2001.
doi: 10.1523/Jneurosci.21-08-02580.2001 pmid: 11306611 |
| 31. |
Ekdahl CT, Claasen JH, Bonde S, et al. Inflammation is detrimental for neurogenesis in adult brain. P Natl Acad Sci U S A 2003; 100(23):13632-7. doi: 10.1073/pnas.2234031100.
doi: 10.1073/pnas.2234031100 |
| 32. |
O’Connor JC, Lawson MA, Andre C, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 2009; 14(5):511-22. doi: 10.1038/sj.mp.4002148.
doi: 10.1038/sj.mp.4002148 |
| 33. |
Frenois F, Moreau M, O’Connor J, et al. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology 2007; 32(5):516-31. doi: 10.1016/j.psyneuen.2007.03.005.
doi: 10.1016/j.psyneuen.2007.03.005 |
| 34. |
Tynan RJ, Naicker S, Hinwood M, et al. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun 2010; 24:1058-68. doi: 10.1016/j.bbi.2010.02.001.
doi: 10.1016/j.bbi.2010.02.001 pmid: 20153418 |
| 35. |
Han RR, Liu ZY, Sun NN, et al. BDNF alleviates neuroinflammation in the hippocampus of type 1 diabetic mice via blocking the aberrant HMGB1/RAGE/NF-kappa B pathway. Aging Dis 2019; 10(3):611-25. doi: 10.14336/Ad.2018.0707.
doi: 10.14336/Ad.2018.0707 |
| 36. |
Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 2009; 27:119-45. doi: 10.1146/annurev.immunol.021908.132528.
doi: 10.1146/annurev.immunol.021908.132528 pmid: 19302036 |
| 37. |
Kettenmann H, Hanisch UK, Noda M, et al. Physiology of microglia. Physiol Rev 2001; 91(2):461-553. doi: 10.1152/physrev.00011.2010.
doi: 10.1152/physrev.00011.2010 |
| 38. |
Konsman JP, Kelley K, Dantzer R. Temporal and spatial relationships between lipopolysaccharide-induced expression of fos, interleukin-1 beta and inducible nitric oxide synthase in rat brain. Neuroscience 1999; 89(2):535-548. doi: 10.1016/S0306-4522(98)00368-6.
doi: 10.1016/S0306-4522(98)00368-6 pmid: 10077334 |
| 39. |
Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res 2010; 1350:18-34. doi: 10.1016/j.brainres.2010.03.059.
doi: 10.1016/j.brainres.2010.03.059 pmid: 20353764 |
| 40. |
Kawai Y. Differential ascending projections from the male rat caudal nucleus of the tractus solitarius: an interface between local microcircuits and global macrocircuits. Front Neuroanat 2018; 12:63. doi: 10.3389/Fnana.2018.00063.
doi: 10.3389/Fnana.2018.00063 |
| 41. |
Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat RevNeurosci 2009; 10(6):397-409. doi: 10.1038/nrn2647.
doi: 10.1038/nrn2647 |
| 42. |
Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci 2013; 14(9):609-25. doi: 10.1038/nrn3381.
doi: 10.1038/nrn3381 |
| 43. |
Michelsen KA, Schmitz C, Steinbusch HWM. The dorsal raphe nucleus: from silver stainings to a role in depression. Brain Res Rev 2007; 55(2):329-342. doi: 10.1016/j.brainresrev.2007.01.002.
doi: 10.1016/j.brainresrev.2007.01.002 pmid: 17316819 |
| 44. |
George DT, Ameli R, Koob GF. Periaqueductal gray sheds light on dark areas of psychopathology. Trends Neurosci 2019; 42(5):349-60. doi: 10.1016/j.tins.2019.03.004.
doi: S0166-2236(19)30038-4 pmid: 30955857 |
| 45. |
Schwarz LA, Luo LQ. Organization of the locus coeruleus-norepinephrine system. Curr Biol 2015; 25(21):R1051-R1056, doi: 10.1016/j.cub.2015.09.039.
doi: 10.1016/j.cub.2015.09.039 |
| 46. |
Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev 2000; 24(1):85-105. doi: 10.1016/S0149-7634(99)00065-2.
doi: 10.1016/S0149-7634(99)00065-2 pmid: 10654664 |
| 47. |
Konsman JP, Luheshi GN, Bluthe RM, et al. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals: a functional anatomical analysis. Eur J Neurosci 2000; 12:4434-46. doi: 10.1046/j.0953-816X.2000.01319.x.
doi: 10.1046/j.0953-816X.2000.01319.x pmid: 11122354 |
| 48. |
Sarhan M, Freund-Mercier MJ, Veinante P. Branching patterns of parabrachial neurons projecting to the central extended amgydala: Single axonal reconstructions. J Comp Neurol 2005; 491(4):418-42. doi: 10.1002/cne.20697.
doi: 10.1002/cne.20697 pmid: 16175547 |
| 49. |
Gaykema RPA, Chen CC, Goehler LE. Organization of immune-responsive medullary projections to the bed nucleus of the stria terminalis, central amygdala, and paraventricular nucleus of the hypothalamus: evidence for parallel viscerosensory pathways in the rat brain. Brain Res 2007; 1130(1):130-45. doi: 10.1016/j.brainres.2006.10.084.
doi: 10.1016/j.brainres.2006.10.084 pmid: 17169348 |
| 50. |
Marvel FA, Chen CC, Badr N, et al. Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-Fos expression in central autonomic nuclei. Brain Behav Immun 2004; 18:123-34. doi: 10.1016/j.bbi.2003.09.004.
doi: 10.1016/j.bbi.2003.09.004 |
| 51. |
Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci 1994; 14(2):897-913. doi: 10.1523/JNEUROSCI.14-02-00897.1994.
doi: 10.1523/JNEUROSCI.14-02-00897.1994 pmid: 8301368 |
| 52. |
Van Bockstaele EJ, Peoples J, Telegan P. Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: evidence for a monosynaptic pathway. J Comp Neurol 1999; 412(3):410-28. doi: 10.1002/(Sici)1096-9861(19990927)412:3<410::Aid-Cne3>3.0.Co;2-F.
doi: 10.1002/(Sici)1096-9861(19990927)412:3<410::Aid-Cne3>3.0.Co pmid: 10441230 |
| 53. |
Borsody MK, Weiss JM. The effects of endogenous interleukin-1 bioactivity on locus coeruleus neurons in response to bacterial and viral substances. Brain Res 2004; 1007(1-2):39-56. doi: 10.1016/j.brainres.2004.02.011.
doi: 10.1016/j.brainres.2004.02.011 pmid: 15064134 |
| 54. |
Borsody MK, Weiss JM. The subdiaphragmatic vagus nerves mediate activation of locus coeruleus neurons by peripherally administered microbial substances. Neuroscience 2005; 131(1):235-45. doi: 10.1016/j.neuroscience.2004.09.061.
doi: 10.1016/j.neuroscience.2004.09.061 pmid: 15680706 |
| 55. |
Chen LW, Rao ZR, Shi JW. Catecholaminergic neurons in the nucleus-tractus-solitarii which send their axons to the midbrain periaqueductal gray express fos protein after noxious-stimulation of the stomach: a triple labeling study in the rat. Neurosci Lett 1995; 189(3):179-81. doi: 10.1016/0304-3940(95)11475-C.
doi: 10.1016/0304-3940(95)11475-C pmid: 7624039 |
| 56. |
Bhatt S, Bhatt RS, Zalcman SS, et al. Peripheral and central mediators of lipopolysaccharide induced suppression of defensive rage behavior in the cat. Neuroscience 2009; 163(4):1002-11. doi: 10.1016/j.neuroscience.2009.07.050.
doi: 10.1016/j.neuroscience.2009.07.050 pmid: 19647047 |
| 57. |
Floyd NS, Keay KA, Arias CM, et al. Projections from the ventrolateral periaqueductal gray to endocrine regulatory subdivisions of the paraventricular nucleus of the hypothalamus in the rat. Neurosci Lett 1996; 220(2):105-8. doi: 10.1016/S0304-3940(96)13240-7.
doi: 10.1016/S0304-3940(96)13240-7 pmid: 8981484 |
| 58. |
Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brain-stem to the paraventricular and supraoptic nuclei in the rat. Brain Res Rev 1982; 257(3):275-325. doi: 10.1016/0165-0173(82)90010-8.
doi: 10.1016/0165-0173(82)90010-8 |
| 59. |
Bienkowski MS, Rinaman L. Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic-pituitary-adrenal axis and central fos activation in rats after acute systemic endotoxin exposure. Neuroscience 2008; 156(4):1093-2. doi: 10.1016/j.neuroscience.2008.08.011.
doi: 10.1016/j.neuroscience.2008.08.011 pmid: 18773942 |
| 60. |
Herman JP. Regulation of hypothalamo-pituitary-adrenocortical responses to stressors by the nucleus of the solitary tract/dorsal vagal complex. Cell Mol Neurobiol 2018; 38(1):25-35. doi: 10.1007/s10571-017-0543-8.
doi: 10.1007/s10571-017-0543-8 pmid: 28895001 |
| 61. |
Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a review and analysis of alternative mechanisms. Life Sci 1995; 57(11):1011-26. doi: 10.1016/0024-3205(95)02047-M.
doi: 10.1016/0024-3205(95)02047-m pmid: 7658909 |
| 62. |
Buller KM. Role of circumventricular organs in pro-inflammatory cytokine-induced activation of the hypothalamic-pituitary-adrenal axis. Clin Exp Pharmacol Physiol 2001; 28(7):581-9. doi: 10.1046/j.1440-1681.2001.03490.x.
doi: 10.1046/j.1440-1681.2001.03490.x |
| 63. |
Shapiro RE, Miselis RR. The central neural connections of the area postrema of the rat. J Comp Neurol 1985; 234(3):344-64. doi: 10.1002/cne.902340306.
doi: 10.1002/cne.902340306 pmid: 3988989 |
| [1] | Ran Li,Zhanyun Lv,Yanxin Li,Wei Li,Yanlei Hao. Effects of TYROBP Deficiency on Neuroinflammation of a Alzheimer’s Disease Mouse Model Carrying a PSEN1 p.G378E Mutation [J]. Chinese Medical Sciences Journal, 2022, 37(4): 320-330. |
| [2] | Xue Zhang, Yan Xu, Lijian Pei. Review of Neuromyelitis Optica Spectrum Disorder with Pain-Depression Comorbidity [J]. Chinese Medical Sciences Journal, 2021, 36(4): 316-322. |
| [3] | Cai Cui,Xu Changqing,Jin Hualiang,Li Bei. Combined Effects of Chronic Obstructive Pulmonary Disease and Depression on Spatial Memory in Old Rats [J]. Chinese Medical Sciences Journal, 2018, 33(4): 260-266. |
| [4] | Pan Yanfang, Jia Xiaotao, Song Erfei, Peng Xiaozhong. Astragaloside IV Protects Against Aβ1-42-induced Oxidative Stress, Neuroinflammation and Cognitive Impairment in Rats [J]. Chinese Medical Sciences Journal, 2018, 33(1): 29-37. |
| [5] | Gao Zeng, Jie Liu, Ning Wu, Cong-wei Jia, Shu-bin Guo. Lipopolysaccharide Challenge Induces Long Pentraxin 3 Expression in Mice Independently from Acute Lung Injury [J]. Chinese Medical Sciences Journal, 2015, 30(1): 7-17. |
| [6] | Yu-ping Mo,Hai-jiang Yao,Hong-tao Song,An-ping Xu,Yin-shan Tang,Zhi-gang Li*. Progress of Animal Research on Electro-acupuncture Treatment for Depression [J]. Chinese Medical Sciences Journal, 2014, 29(1): 43-47. |
| [7] | Kang-sheng Liu, Jia-hu Hao, Yu-qing Xu, Xiao-qi Gu, Juan Shi, Chun-fang Dai, Fei Xu, and Rong Shen. Breast Milk Lead and Cadmium Levels in Suburban Areas of Nanjing, China [J]. Chinese Medical Sciences Journal, 2013, 28(1): 7-15. |
| [8] | Wen-min Tian, Ke-rang Zhang, Juan Zhang, Yan Shen and Qi Xu. Association between the Epidermal Growth Factor Gene and Intelligence in Major Depression Patients [J]. Chinese Medical Sciences Journal, 2010, 25(2): 105-108. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|



