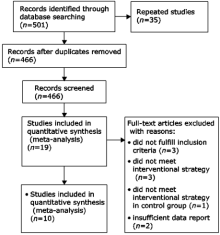
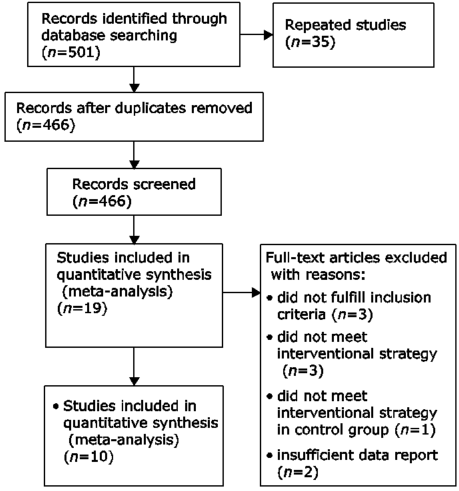
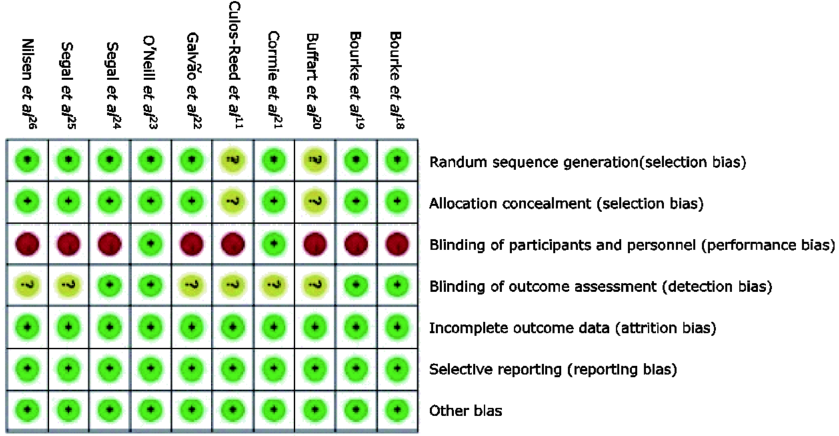
Chinese Medical Sciences Journal ›› 2017, Vol. 32 ›› Issue (1): 13-21.doi: 10.24920/J1001-9242.2007.002
• Orginal Article • Previous Articles Next Articles
Effects of Exercise on Cancer-related Fatigue and Quality of Life in Prostate Cancer Patients Undergoing Androgen Deprivation Therapy: A Meta-analysis of Randomized Clinical Trials
- Department of Urology, Xixi Hospital of Hangzhou, Hangzhou 310023, China
-
Published:2017-03-31Online:2017-04-10 -
Contact:Yang Bo E-mail:xxyyyangbo@aliyun.com
Cite this article
Yang Bo, Wang Jiansheng. Effects of Exercise on Cancer-related Fatigue and Quality of Life in Prostate Cancer Patients Undergoing Androgen Deprivation Therapy: A Meta-analysis of Randomized Clinical Trials[J].Chinese Medical Sciences Journal, 2017, 32(1): 13-21.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 1
Characteristics of included studie"
| Studies | Sample size | Mean age§ (yrs) | ADT status | Exercise interventions | Control interventions | Outcome measures | Follow-up |
|---|---|---|---|---|---|---|---|
| Bourke et al18 | Exercise: 25 Control: 25 | 71.3±6.4 72.2±7.7 | ≥6 months ADT | 12-week lifestyle intervention combined with supervised and self-directed exercise with dietary advice | Usual care | Fatigue [FACT-F] QoL [FACT-P] | 12 weeks |
| Bourke et al19 | Exercise: 43 Control: 43 | 71±6 71±8 | ≥6 months ADT | 12-week lifestyle intervention consisting of aerobic and resistance exercise with parallel dietary advice | Usual care | Fatigue [FACT-F] QoL [FACT-P] | 12 weeks |
| Buffart et al20 | Exercise: 29 Control: 28 | 69.7±7.3 70.1±7.3 | ≥2 months ADT | 12-week lifestyle intervention consisting of aerobic and resistance exercise (program of chest press, seated row, shoulder press, triceps extension, leg press, leg extension, leg curl, and abdominal crunch) | Usual care | Fatigue [EORTC, QLQ-C30] QoL [SF-36] | 12 weeks |
| Cormie et al21 | Exercise: 32 Control: 31 | 69.6±6.5 67.1±7.5 | ≥3 months ADT | Twice weekly exercise sessions for 3 months consisting of an aerobic and resistance exercise program with parallel dietary advice | Usual care | Fatigue [FACT-F] QoL [SF-36] | 3 months |
| Culos-Reed et al11 | Exercise: 53 Control: 476 | 67.2±8.8 68.0±8.4 | ≥6 months ADT | 16-week supervised and unsupervised program of walking, stretching, light resistance and core strengthening | Usual care | Fatigue [FSS] QoL [EORTC-QOLC-30] | 16 weeks |
| Galvão et al22 | Exercise: 29 Control: 28 | 69.5±7.3 70.1±7.3 | ≥2 months ADT, Continuing for subsequent 6 months | 12-week combined progressive resistance and aerobic training | Usual care | QoL [SF-36] | 12 weeks |
| Studies | Sample size | Mean age§ (yrs) | ADT status | Exercise interventions | Control interventions | Outcome measures | Follow-up |
| O’Neill et al23 | Exercise: 47 Control: 47 | 69.7±6.8 69.9±7.0 | ≥6 months ADT | Walk at a brisk pace for at least 30 minutes per day, five or more days per week in line with UK physical activity guidelines for 6 months, and consume a diet commensurate with UK healthy eating guidelines | Usual care | Fatigue [FSS] QoL [FACT-P] | |
| Segal et al24 | Exercise: 82 Control: 73 | 68.2±7.9 67.7±7.5 | ≥3 months ADT | 12-week program of 9 strength training exercises carried out under supervision 3 times per week | Usual care | Fatigue [FACT-F] QoL [FACT-P] | 12 weeks |
| Segal et al25 | Resistance exercise: 40 Aerobic exercise: 40 Control: 41 | 66.4±7.6 66.2±6.8 65.3±7.6 | ≥16 weeks ADT | Resistance exercise: 24-week program of three sessions per week performing two sets of 8-12 repetitions of 10 different exercises Aerobic exercise: 24-week program of three sessions per week with a cycle ergometer, treadmill, or elliptical trainer, beginning at 50% to 60% of the predetermined peak oxygen consumption (VO2peak) for weeks 1 to 4, progressing to 70%-75% for weeks 5 to 24 | Usual care | Fatigue [FACT-F] QoL [FACT-G] | 24 weeks |
| Nilsen et al26 | Exercise: 28 Control: 30 | 66 (range, 54-76) 66 (range, 54-76) | 2-6 months after the initiation of neo-adjuvant ADT, followed by 9-36 months adjuvant ADT | 16-week program of 9 strength training exercises carried out under supervision 3 times per week | Usual care | Fatigue [EORTC-QOLC-30] QoL [EORTC-QOLC-30] | 16 weeks |
Table 3
Results of sensitivity analysis"
| Omitting study | CRF (fixed model) | QoL (fixed model) | |
|---|---|---|---|
| SMD (95% CI), P heterogeneity, I2, n | SMD (95% CI), P heterogeneity, I2, n | ||
| Bourke et al18 | -0.28 (-0.42 to -0.14), 0.32, 14%, 734 | 0.20 (0.07 to 0.34), 0.53, 0, 791 | |
| Bourke et al19 | -0.31 (-0.45 to -0.16), 0.09, 41%, 698 | 0.18 (0.04 to 0.32), 0.68, 0, 755 | |
| Buffart et al20 | -0.27 (-0.41 to -0.13), 0.48, 0, 727 | 0.19 (0.05 to 0.33), 0.63, 0, 784 | |
| Cormie et al21 | -0.32 (-0.46 to -0.17), 0.09, 42%, 721 | 0.24 (0.1 to 0.38), 0.81, 0, 778 | |
| Culos-Reed et al11 | -0.33 (-0.48 to -0.18), 0.09, 41%, 684 | 0.20 (0.06 to 0.34), 0.51, 0, 741 | |
| Galvão et al22 | - | 0.19 (0.05 to 0.33), 0.63, 0, 784 | |
| O’Neill et al23 | -0.32 (-1.47 to -0.17), 0.09, 42%, 690 | 0.23 (0.09 to 0.37), 0.60, 0, 747 | |
| Segal et al24 | -0.36 (-0.51 to -0.21), 0.14, 34%, 629 | 0.22 (0.07 to 0.36), 0.51, 0, 686 | |
| Segal et al25 | -0.32 (-0.48 to -0.17), 0.06, 49%, 663 | 0.22 (0.07 to 0.36), 0.41, 2%, 720 | |
| Nilsen et al26 | -0.34 (-0.49 to -0.20), 0.15, 33%, 726 | 0.22 (0.08 to 0.36), 0.55, 0, 783 | |
| 1. | Brown JC, Huedo-Medina TB, Pescatello LS, et al.Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2011; 20:123-33. |
| 2. | Janda M, Gerstner N, Obermair A, et al.Quality of life changes during conformal radiation therapy for prostate carcinoma. Cancer 2000; 89:1322-8. |
| 3. | Kruijsen-Jaarsma M, Révész D, Bierings MB, et al.Effects of exercise on immune function in patients with cancer: a systematic review. Exerc Immunol Rev 2013; 19:120-43. |
| 4. | Betof AS, Dewhirst MW, Jones LW.Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain Behav Immun 2013; 30:S75-87. |
| 5. | Courneya KS, Segal RJ, McKenzie DC, et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc 2014; 46:1744-51. |
| 6. | Victorson D, Barocas J, Song J, et al.Reliability across studies from the functional assessment of cancer therapy-general (FACT-G) and its subscales: a reliability generalization. Qual Life Res 2008; 17:1137-46. |
| 7. | Baade PD, Youlden DR, Cramb SM, et al.Epidemiology of prostate cancer in the Asia-Pacific region. Prostate Int 2013; 1:47-58. |
| 8. | Schroeder A, Herrmann A, Cherryholmes G, et al.Loss of androgen receptor expression promotes a stem-like cell phenotype in prostate cancer through STAT3 signaling. Cancer Res 2014; 74:1227-37. |
| 9. | Alberga AS, Segal RJ, Reid RD, et al.Age and androgen- deprivation therapy on exercise outcomes in men with prostate cancer. Support Care Cancer 2012; 20:971-81. |
| 10. | Shahinian VB, Kuo Y-F, Freeman JL, et al.Risk of the “androgen deprivation syndrome” in men receiving androgen deprivation for prostate cancer. Arch Intern Med 2006; 166:465-71. |
| 11. | Culos-Reed SN, Robinson JW, Lau H, et al.Physical activity for men receiving androgen deprivation therapy for prostate cancer: benefits from a 16-week intervention. Support Care Cancer 2010; 18:591-9. |
| 12. | Bourke L, Doll H, Crank H, et al.Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: a feasibility study. Cancer Epidemiol Biomarkers Prev 2011; 20:647-57. |
| 13. | Keogh JW, MacLeod RD. Body composition, physical fitness, functional performance, quality of life, and fatigue benefits of exercise for prostate cancer patients: a systematic review. J Pain Symptom Manage 2012; 43: 96-110. |
| 14. | Winters-Stone KM, Beer TM.Review of exercise studies in prostate cancer survivors receiving androgen deprivation therapy calls for an aggressive research agenda to generate high-quality evidence and guidance for exercise as standard of care. J Clin Oncol 2014; 32:2518-9. |
| 15. | Liberati A, Altman DG, Tetzlaff J, et al.The PRISMA statement for reporting systematic reviews and meta- analyses of studies that evaluate health care interven- tions: explanation and elaboration. Ann Intern Med 2009; 151:W65-94. |
| 16 | 16.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions Version 5.1.0 [updated March2011]. The Cochrane Collaboration. Available from: |
| 17. | Higgins JP, Thompson SG, Deeks JJ, et al.Measuring inconsistency in meta-analyses. BMJ 2003; 327:557-60. |
| 18. | Bourke L, Doll H, Crank H, et al.Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: a feasibility study. Cancer Epidemiol Biomarkers Prev 2011; 20:647-57. |
| 19. | Bourke L, Gilbert S, Hooper R, et al.Lifestyle changes for improving disease-specific quality of life in sedentary men on long-term androgen-deprivation therapy for advanced prostate cancer: a randomised controlled trial. Eur Urol 2014; 65:865-72. |
| 20. | Buffart LM, Galvão DA, Mai JC, et al.Mediators of the resistance and aerobic exercise intervention effect on physical and general health in men undergoing androgen deprivation therapy for prostate cancer. Cancer 2014; 120:294-301. |
| 21. | Cormie P, Galvão DA, Spry N, et al.Can supervised exercise prevent treatment toxicity in prostate cancer patients initiating androgen deprivation therapy: a randomised controlled trial. BJU International 2014; 115:256-66. |
| 22. | Galvão DA, Taaffe DR, Spry N, et al.Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol 2010; 28:340-7. |
| 23. | O’Neill RF, Haseen F, Murray LJ. A randomised controlled trial to evaluate the efficacy of a 6-month dietary and physical activity intervention for patients receiving androgen deprivation therapy for prostate cancer. J Cancer Surviv 2015; 9:431-40. |
| 24. | Segal RJ, Reid RD, Courneya KS, et al.Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 2003; 21:1653-9. |
| 25. | Segal RJ, Reid RD, Courneya KS, et al.Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol 2009; 27:344-51. |
| 26. | Nilsen TS, Raastad T, Skovlund E, et al.Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncologica 2015; 54:1-9. |
| 27. | Floyd A, Moyer A.Group versus individual exercise interventions for women with breast cancer: a meta- analysis. Health Psychol Rev 2010; 4:22-41. |
| 28. | Sherrington C, Whitney JC, Lord SR, et al.Effective exercise for the prevention of falls: a systematic review and meta-analysis. J Am Geriatr Soc 2008; 56:2234-43. |
| 29. | Gardner JR, Livingston PM, Fraser SF.Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol 2014; 32:335-46. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|